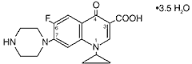
Telmisartan Ip Micr - Pharmaceutical Substance, Angiotensin Ii Receptor Blocker, Ensured Purity, Consistent Potency , Microbial & Heavy Metal Safe, Uniform Dosage
Price: 1800 INR / Kilograms
Get Latest Price
Minimum Order Quantity :
25 Kilograms
Brand Name :
Telmisartan Ip Micr.
In Stock
Product Specifications
| Place of Origin | India |
| Storage | Other, Store in a cool, dry place, protected from light |
| Loss on Drying | a%0$?0.5% |
| Assay | Not less than 99% |
| Particle Size | Micronized |
| Molecular Formula | C33H30N4O2 |
| Residue on Ignition | a%0$?0.1% |
| HS Code | 29335990 |
| Heavy Metal (%) | a%0$?0.001% |
| Moisture (%) | a%0$?0.5% |
| Melting Point | 261AdegC - 263AdegC |
| Molecular Weight | 514.63 |
| Other Names | Telmisartan |
| CAS No | 144701-48-4 |
| Type | Other, Active Pharma Ingredient (API) |
| Grade | Other, Pharmaceutical Grade |
| Usage | Pharmaceutical, Antihypertensive agent |
| Purity | a%0Y=99% |
| Appearance | Solid |
| Application | Other, Active Pharmaceutical Ingredient, Anti-hypertensive medication |
| Raw Material | Yes |
| Smell | Other, Odorless |
| Color | Other, White to off-white |
| Form | Powder |
Product Overview
Key Features
Assay: Quantitative determination of the potency of Telmisartan, typically expressed as a percentage of the labeled amount, ensuring consistency in therapeutic efficacy.
Related Substances: Limits on impurities and degradation products to maintain purity and safety.
Residual Solvents: Maximum allowable levels of solvents used in manufacturing, ensuring the final product is free from harmful residues.
Loss on Drying: Measurement of moisture content to prevent degradation and ensure stability throughout storage and use.
Heavy Metals: Strict limits on toxic heavy metals such as lead, arsenic, mercury, and cadmium to ensure product safety.
Microbial Limits: Specifications ensuring the absence of harmful microorganisms, maintaining microbiological purity.
Particle Size Distribution: Requirements for particle size uniformity to optimize dissolution and absorption, enhancing bioavailability.
Uniformity of Dosage Units: Criteria ensuring consistent dosage across individual tablets or capsules, crucial for treatment efficacy.
Packaging: Specifications for materials and labeling that preserve the integrity and stability of Telmisartan during storage and distribution.
Company Details
Business Type
Manufacturer, Supplier, Trading Company
Employee Count
50
Establishment
2018
Working Days
Monday To Saturday
GST NO
27AAHFI0743H1ZH
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 27AAHFI0743H1ZH
Mumbai, Maharashtra
Marketing Executive
Ms Pooja S
Members since
5 Years
Address
B No. 217, Champaklal Industrial Estate, 2nd Floor, Sion East, Mumbai, Maharashtra, 400022, India
active pharmaceutical ingredients in Mumbai
Report incorrect details