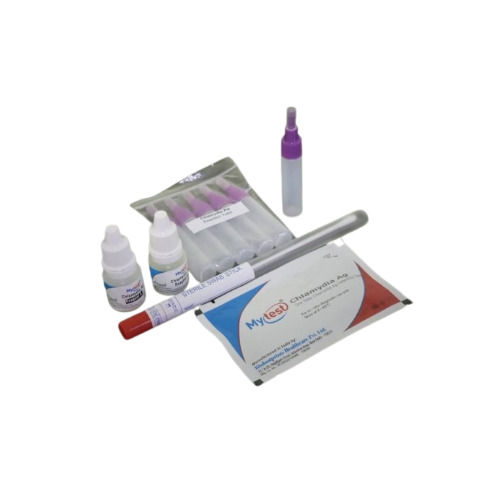
Mytest Chlamydia Ag Rapid Test Kit - Easy-to-use, Portable Device With High Sensitivity And Specificity, Ce And Fda Approved
Price:
Get Latest Price
In Stock
Product Specifications
| Condition | New |
| Portable | Yes |
| Color | White |
| Attributes | Easy To Operate |
| Usage | Used for rapid test |
| Payment Terms | Telegraphic Transfer (T/T) |
| Delivery Time | 1 Days |
| Packaging Details | Carton Box |
| Main Domestic Market | All India |
Product Overview
Key Features
Contents of The Kit
Each Foil Pouch Contains:-
Mytest Chlamydia Ag Device individually foil pouched with a desiccant
Extraction Buffer
Sample Applicator (Optional)
Package insert (instruction for use)
Rapid results in minutes.
High sensitivity and specificity.
User-friendly with simple instructions.
Portable and easy to use.
Reliable detection of Chlamydia antigen.
Suitable for professional and at-home use.
Convenient sample collection methods.
CE and FDA approved for accuracy.
Shelf-stable components for extended storage.
Company Details
Bio Footprints Healthcare Pvt Ltd an IVD/Diagnostics kits manufacturer based at New Delhi. Bio Footprints is owned, fueled, managed and propelled by a young and vibrant team, hailing from Indian IVD Industry. Our products are sold under our registered brand name âÂA ÂA My testâÂA ÂA . We currently manufacture more than 33 types of Rapid tests for diagnosing Infectious, Febrile, Gastrointestinal, Respiratory, Cancer, Covid -19 and other related sicknesses. In our ISO 13485 & CE certified, state of the art facility we develop the best quality in-vitro diagnostics products which contribute to our national algorithms and global needs in diagnosis of chronic and communicable diseases. Product and Services Rapid Diagnostics Tests manufacturing OEM and Contract manufacturing Research and Development
Business Type
Exporter, Manufacturer, Distributor, Supplier, Trading Company, Wholesaler
Employee Count
15
Establishment
2013
Working Days
Monday To Sunday
GST NO
07AAFCB4261Q1ZA
Certification
ISO13485 & 9001
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AAFCB4261Q1ZA
New Delhi, Delhi
Director
Ms Kiran
Address
70/A-29, Najafgarh, Rama Road, Industrial Area, New Delhi, Delhi, 110015, India
Report incorrect details