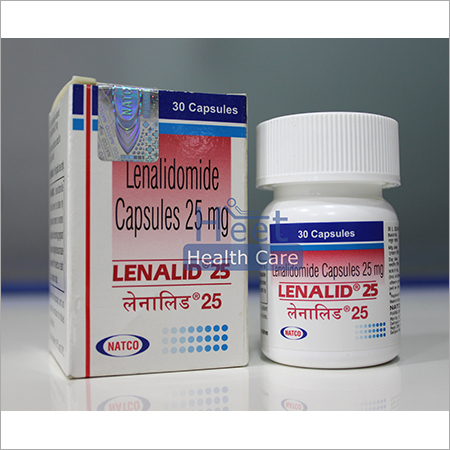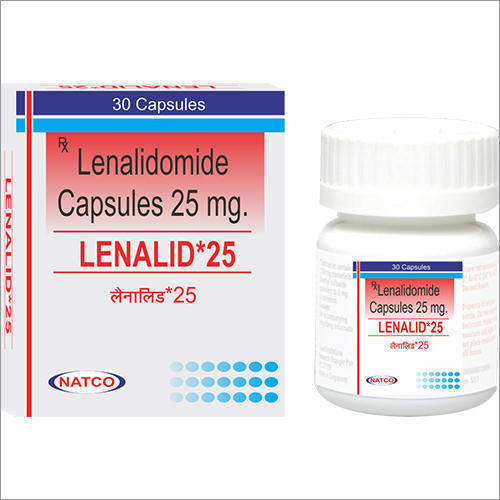
Lenalid Capsules - 25mg Dosage, 30 Capsules | Active Ingredient: Lenalidomide, Treatment For Multiple Myeloma And Mantle Cell Lymphoma
Price: 2000.00 INR / Box
(2000.00 INR + 0% GST)
Get Latest Price
1 Pack Contains :
1
Minimum Pack Size :
5
In Stock
Product Specifications
| Dosage | 25mg |
| Form | Capsule |
| Packaging | 30 Capsules |
| Manufacturer | Natco |
| Active Ingredient | Lenalidomide |
| Usage | Treatment of multiple myeloma and mantle cell lymphoma, as directed by a physician. |
| Regulatory Compliance | Pharmacopeia standards |
| Features | Cancer treatment, Oral dosage, 25mg capsules, Convenient use, Effective therapy |
| FOB Port | Ahmedabad |
| Payment Terms | Cash in Advance (CID), Cash Advance (CA) |
| Supply Ability | 1000 Per Week |
| Delivery Time | 1 Week |
| Sample Available | Yes |
| Sample Policy | Within a certain price range free samples are available |
| Packaging Details | 30 capsules in 1 strip |
| Main Export Market(s) | Western Europe, Australia, North America, Eastern Europe, Middle East, Central America, South America, Asia, Africa |
| Product Unit | 5 Piece/Pieces |
| Moq | 5 |
| Stock Quantity | 25 |
| Packsize | 1 |
| Price Type | fixed |
| Brand Name | Natco Pharma Ltd |
| Price | 2000.00 INR (Approx.) |
| Mop | 5 |
| Returnable | No |
| Unit Type | Piece/Pieces |
| Currency | INR |
| Minimum Order Quantity | 5 |
| Minimum Ordered Packs | 5 |
| GSTIN | 0% |
Company Details
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
80
Establishment
1998
Working Days
Monday To Saturday
GST NO
24ABMPB3030G1ZA
Payment Mode
Cash Advance (CA)
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24ABMPB3030G1ZA
Ahmedabad, Gujarat

Accepts only Foreign inquiries
Chief Executive Officer
Mr Rakesh Bhattacharjee
Members since
18 Years
Address
West Port, 16th Floor, beside: - Taj Skyline Hotel, Sindhu Bhavan Road, Near S.G Highway, Thaltej, Ahmedabad, Gujarat, 380059, India
lenalid capsules in Ahmedabad
Report incorrect details