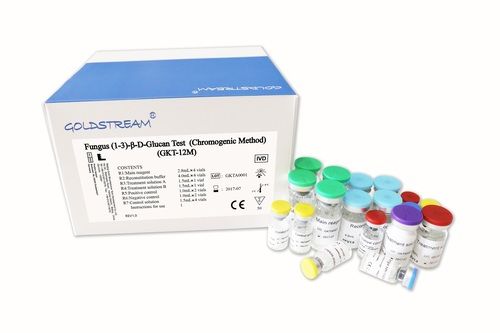
Fungus (1-3)-i -D-glucan Test Kit I Chromogenic Methodi
Price:
Get Latest Price
In Stock
Product Specifications
| Model | GKT-12M |
| Tests/kit | 36 |
| Reagents | 4 vials |
| Detection time | 60 min |
| Reader | Microplate Reader |
| Sample type | Serum, BAL fluid |
| Linear range | 31.25-500 pg/ml |
| Features | Fast results, Accurate detection, Easy to use, High sensitivity, Specific assay, Reliable results, Chromogenic method |
Product Overview
Key Features
Specification : 110 tests/kit, 50 tests/kit, 50 tests/kit, 36 tests/kit, 30 tests/kit
Number of main reagents : 2 vials, 2 vials, 4 vials, 3 vials, 6 vials
Detection time : 40 min60 min
Instrument : Microplate ReaderKinetic Tube Reader
Method : Chromogenic Method
Sample type : Serum and BAL fluid
Linear range : 31.25-500 pg/ml
Specificity : The cross-reaction rate of 100 pg/ml endotoxin solution is less than 2%
Inter assay variation : a 10%
Company Details
Business Type
Manufacturer
Employee Count
200
Establishment
1997
Working Days
Monday To Sunday
Payment Mode
Telegraphic Transfer (T/T)
Related Products
Explore Related Categories
More Product From This seller
Seller Details
Tianjin, Tianjin
Ms Amy Zhang
Address
Xinuo, Anzheng Road, Tourism Area, Tianjin, Tianjin, China
diagnostic apparatus in Tianjin
Report incorrect details