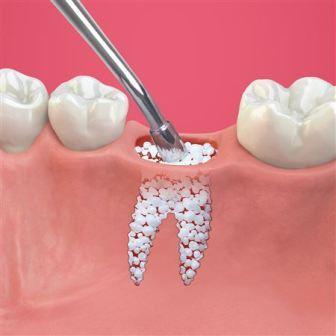
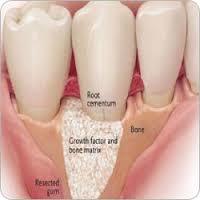
LINACOL MEDICAL CureOs BLOCKS BONE GRAFT During the design and production of the product, it was ensured that the interconnected pore structure, in which the bone cells can be placed, is considered. The balance between bone construction and destruction shifts in the direction of destruction in the so-called osteoporosis diseases with aging. The artificial bone tissue is used to strengthen the bone construction in the osteoporosis disease which decreases the mineral density of the bone and increases the fragility with the changes in the microstructure. Bone tissue is controlled by the blood-based regulators, the physical environment, the neural network surrounding the perioosteum, and the genetic structure of the person. Developed artificial bone tissue is designed to allow this control. (Keskin DS, Tezcaner A, Korkusuz P, Korkusuz F, Hasirci V. Collagen-chondroitin sulfate-based PLLA-SAIB-coated rhBMP-2 delivery system for bone repair. Biomaterials 2005;26:4023-34). The balance between construction and destruction is called homeostasis. It can be assumed that construction and destruction has completely renewed in about 4 to 10 years depending on the size of the bone. Unlike other tissues and organs, bone tissue repair is like form of self-renewal, which is called regeneration. The regeneration and repair is not occurred without blood circulation. Therefore, the product is designed to allow renewal of bone and blood circulation as pore diameter and total porosity.