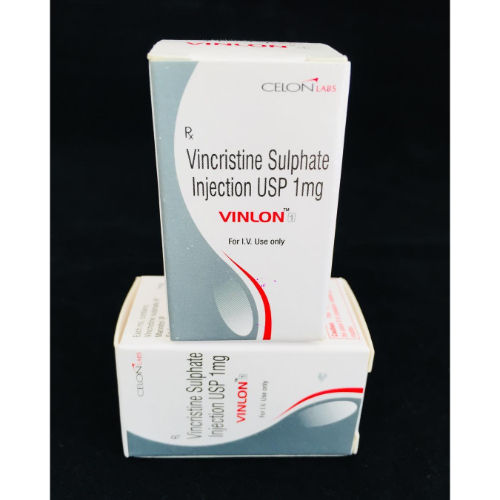
Vincristine Sulfate 1mg Injection - Drug Type: General Medicines
Price: 100 INR / Box
Get Latest Price
Minimum Order Quantity :
100 Box
In Stock
Product Specifications
| Dosage Form | 1mg |
| Indication | Cancer treatment |
| Origin of Medicine | India |
| Drug Type | General Medicines |
| Physical Form | Liquid |
| Function | Cancer Treatment |
| Dosage | As per doctor prescription |
| Suitable For | Adults |
| Quantity | 1 Boxes |
| Payment Terms | Paypal, Cash Advance (CA), Western Union, Others |
| Supply Ability | 1000 Per Month |
| Delivery Time | 5 Days |
| Sample Available | Yes |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | Box |
| Main Domestic Market | Delhi |
Product Overview
Key Features
Targets Cancer Cells
1mg Intravenous Injection
Effective for Leukemia
Used in Lymphomas
Prescription-Only Medication
Company Details
Registered in 2017 , Allumer Medical Pvt. Ltd. has made a name for itself in the list of top suppliers of medical products ,silicone catheter ,disposable syringe in India. The supplier company is located in New Delhi, Delhi and is one of the leading sellers of listed products.Allumer Medical Pvt. Ltd. is listed in Trade India's list of verified sellers offering supreme quality of Double Lumen Catheter Kit ,Sterilized Latex Silicone Catheter ,Advance Syringe etc. Buy medical products ,silicone catheter ,disposable syringe in bulk from us for the best quality products and service.
Business Type
Exporter, Importer, Manufacturer, Service Provider, Distributor, Supplier, Trading Company, Wholesaler, Retailer, Dealer, Fabricator, Producer
Employee Count
100
Establishment
2017
Working Days
Monday To Sunday
GST NO
07AAMCA7785K1ZX
Payment Mode
Paypal
Certification
Gst certificate
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AAMCA7785K1ZX
New Delhi, Delhi

Accepts only Foreign inquiries
Sales Manager
Mr. Hari Shankar
Address
2nd Floor, B- 6/5, Okhla Estate Marg, Okhla Industrial Estate Phase 2, New Delhi, Delhi, 110020, India
Report incorrect details