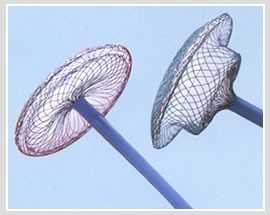
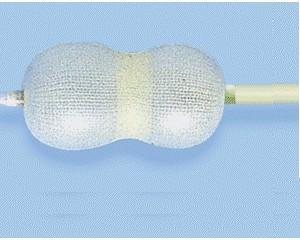
Pda Occluder Device - Feature: Minimally Invasive Alternative To Surgical Closure
Price: 500 USD / Piece
Get Latest Price
Minimum Order Quantity :
50 Piece
In Stock
Product Specifications
| Function | To occlude the patent ductus arteriosus, thereby normalizing blood circulation between the aorta and pulmonary artery |
| Operation Mode | Operated by a trained interventional cardiologist in a catheterization laboratory setting. |
| Feature | Minimally invasive alternative to surgical closure, with a design that facilitates secure placement and endothelialization |
| Instrument | Other, Cardiovascular implant device |
| Material | Steel |
| Condition | New |
| Use Type | Single-use implantable medical device |
| Usage | Intended for the closure of patent ductus arteriosus to prevent abnormal blood flow between the aorta and pulmonary artery |
| Payment Terms | Telegraphic Transfer (T/T) |
| Supply Ability | 50 Per Week |
| Delivery Time | 10 Days |
| Sample Available | Yes |
| Main Export Market(s) | Asia, Australia, Central America, North America |
| Main Domestic Market | All India |
Product Overview
Key Features
Company Details
Xuzhou Yatai Sci-Tech Co.,Ltd., estabilshed in 1999,locates in Xuzhou ,an important industry city in the eastern China.
Yatai is a professional company that produces the third-classifiable intervention medical equipment .There are strong technical power, advanced processes and the company has a complete range of production facilities.The main products include Cardiovascular Balloon Dilatation Catheter ,Occluder Device ,Transport Catheter,Sizing Balloon Catheter,and Cardiac Temporary Pacing Electrode.
Business Type
Exporter, Manufacturer, Supplier, Producer
Employee Count
40
Establishment
1999
Working Days
Monday To Sunday
Certification
ISO 13485,ISO9001
Related Products
Explore Related Categories
More Product From This seller
Seller Details
Xuzhou, Jiangsu Sheng
Mr Frank Meng
Address
Room 204-209, Science & Technology Park Of China University Of Mining And Technology, Xuzhou, Jiangsu Sheng, 221000, China
cardiovascular instrument in Xuzhou
Report incorrect details