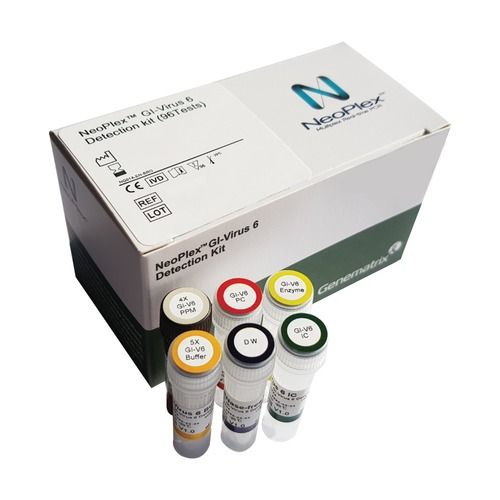
Neoplex Gi-virus 6 Detection Kit
Price:
Get Latest Price
In Stock
Product Specifications
| Condition | New |
| Payment Terms | Telegraphic Transfer (T/T) |
| Delivery Time | 2-3 Week |
| Packaging Details | Box |
Product Overview
Key Features
The 'NeoPlexa c GI-Virus 6 Detection Kit' is a qualitative in vitro test for the simultaneous detection of six gastrointestinal
infection(GI)-causing pathogens including Rotavirus A(RoV), Norovirus GI(NoV GI), Norovirus GII(NoV GII),
Astrovirus(AsV), Adenovirus F (AdV) and Sapovirus(SaV) from stool specimen using one-step based multiplex real-time
RT-PCR. It is an in vitro diagnostic medical device for qualitative examination intended for professionals use.
PRINCIPLE OF ASSAY:
'NeoPlexTM GI-Virus 6 Detection kit' is based on two major processes, isolation of nucleic acid from specimens and
multiplex real-time amplification. Gastrointestinal infection-causing pathogens nucleic acid is extracted from a specimen,
amplified in multiplex One-step real-time RT-PCR and detected using fluorescent reporter dye probes specific for the
pathogen nucleic acid and Internal Control.
Additional required equipment and materials:
-CFX96 real-time PCR detection system (BioRad, Inc., Cat No. 1845097-IVD) or equivalent
-0.2 ml 8-Tube PCR Strips without Caps, low profile, white (BioRad, Inc., Cat No. TLS0851)
-Optical Flat 8-Cap Strips for PCR Tubes (BioRad, Inc., Cat No. TCS0803)
-QIAamp DSP DNA Mini Kit (QIAGEN, Cat No.61304) or equivalent nucleic acid extraction kit
-Pipettes set
-Micro Centrifuge
-Disposable powder-free gloves
Company Details
Business Type
Manufacturer, Distributor, Supplier
Employee Count
64
Establishment
2000
Working Days
Monday To Friday
Payment Mode
Telegraphic Transfer (T/T)
Certification
CE-IVD
Related Products
Explore Related Categories
More Product From This seller
Seller Details
Seongnam, Gyeonggi-do
Mr Daniel Bae
Address
8F, B, Korea Bio Park, 700, Daewangpangyo-ro, Bundang-gu, Seongnam, Gyeonggi-do, 13488, Korea South
rt pcr test kit in Seongnam
Report incorrect details