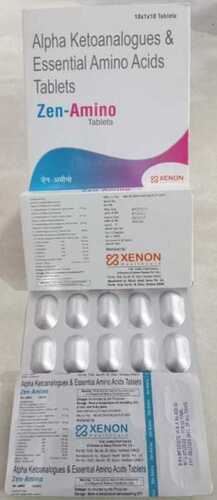
Nefrena Tablet
Price:
Get Latest Price
In Stock
Product Overview
Key Features
I -Ketoanalogue Tablets
a cCalcium-3-methyl-2-oxo-valerate 67 mg
(I -ketoanalogue to isoleucine calcium salt)
a cCalcium-4-methyl-2-oxo-valerate 101 mg
(I -ketoanalogue to leucine calcium salt)
a cCalcium-2-oxo-3-phenylpropionate 68 mg
(I -ketoanalogue to phenylalanine calcium salt)
a cCalcium-3-methyl-2-oxo-butyrate 86 mg
(I -ketoanalogue to valine calcium salt)
a cCalcium-dl-2-hydroxy-4(methylthio) butyrate 59 mg
(I -hydroxyanalogue to methionine calcium salt)
a cLysine acetate 105 mg (Eq to lysine 75 mg.)
a cL-threonine 53 mg
a cL-tryptophan 23 mg
a cL-histidine 38 mg
a cL-tyrosine 30 mg
a cTotal nitrogen content per tablet 36 mg
a cCalcium content per tablet 1.25 mmol=0.05 gm
Dietary protein restriction may improve determinants of CKD progression. However the extent of improvement and effect of ketoanalogue supplementation are unclear. We conducted a prospective randomized controlled trial of safety and efficacy of ketoanaloguea supplemented vegetarian very lowa protein diet (KD) compared with conventional lowa protein diet (LPD). Primary end point was RRT initiation or >50% reduction in initial eGFR. Nondiabetic adults with stable eGFR<30 ml/min per 1.73 m2 proteinuria <1 g/g urinary creatinine good nutritional status and good diet compliance entered a run-in phase on LPD. After 3 months compliant patients were randomized to KD (0.3 g/kg vegetable proteins and 1 cps/5 kg ketoanalogues per day) or continue LPD (0.6 g/kg per day) for 15 months. Only 14% of screened patients patients were randomized with no differences between groups. Adjusted numbers needed to treat (NNTs; 95% confidence interval) to avoid composite primary end point in intention to treat and per-protocol analyses in one patient were 4.4 (4.2 to 5.1) and 4.0 (3.9 to 4.4) respectively for patients with eGFR<30 ml/min per 1.73 m2. Adjusted NNT (95% confidence interval) to avoid dialysis was 22.4 (21.5 to 25.1) for patients with eGFR<30 ml/min per 1.73 m2 but decreased to 2.7 (2.6 to 3.1) for patients with eGFR<20 ml/min per 1.73 m2 in intention to treat analysis. Correction of metabolic abnormalities occurred only with KD. Compliance to diet was good with no changes in nutritional parameters and no adverse reactions. Thus this KD seems nutritionally safe and could defer dialysis initiation in some patients with CKD - Journal of the American Society of Nephrology.
Company Details
Business Type
Supplier, Trading Company
Employee Count
30
Establishment
2003
Working Days
Monday To Sunday
GST NO
07AAECA9312Q1ZA
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AAECA9312Q1ZA
New Delhi, Delhi
Private Limited Company
Mr Aar Ess Remedies
Members since
9 Years
Address
Wz 13e Lane No.4 Vashisth Park Pankha Road New Delhi Delhi 110046 India
Report incorrect details