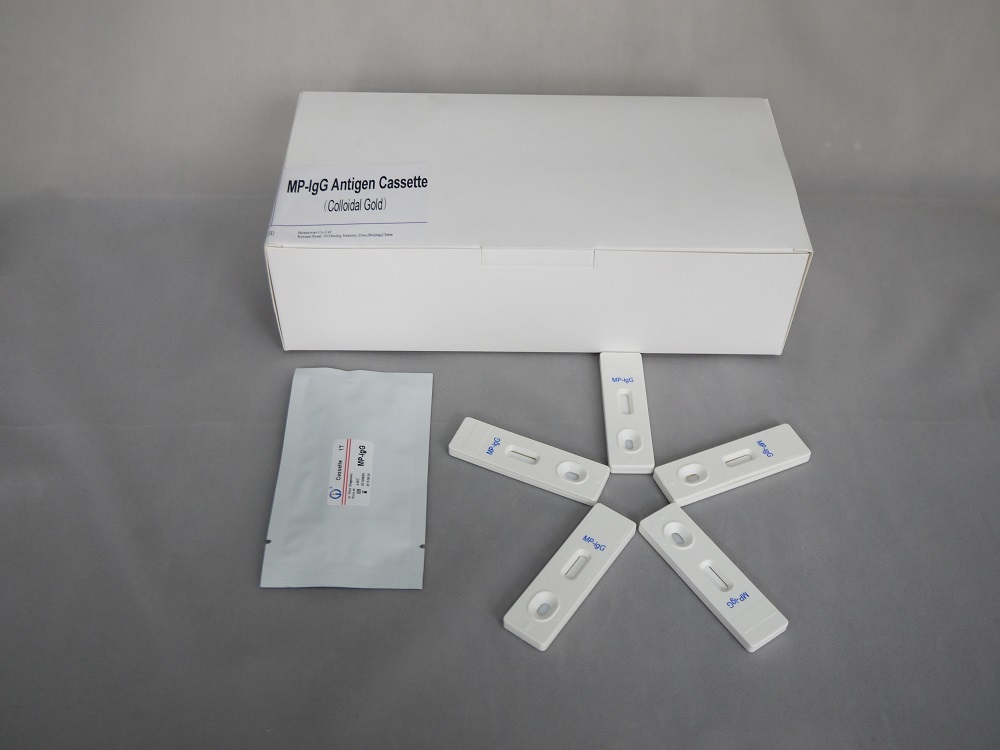
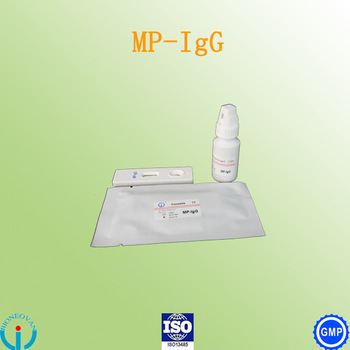




Mycoplasma Pneumoniae(mp)-igg Cassette
Price: 0.9 USD
Get Latest Price
Minimum Order Quantity :
1000
Brand Name :
Bioneovan
In Stock
Product Specifications
| Test cassette dimensions | 40mm x 10mm |
| Sample volume | 10uL |
| Storage temperature | 4-30°C |
| Shelf life | 24 months |
| Test time | 15 minutes |
| Materials | Colloidal gold |
| Packaging | 40 tests/box |
| Features | Rapid results, Easy to use, Accurate detection, High sensitivity, Cost effective, Portable device |
| FOB Port | any port in China |
| Payment Terms | Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Paypal, Others |
| Supply Ability | 1,000,000 Per Month |
| Delivery Time | 15 Days |
| Sample Available | Yes |
| Sample Policy | Free samples available with shipping and taxes paid by the buyer |
| Packaging Details | cartons |
| Main Export Market(s) | Australia, North America, South America, Eastern Europe, Western Europe, Middle East, Central America, Africa, Asia |
| Main Domestic Market | All India |
| Certifications | GMP, ISO9001:2008, SFDA and ISO13485 Certificates. |
Product Overview
Key Features
Company Details
Business Type
Exporter, Manufacturer, Supplier, Trading Company
Employee Count
100
Establishment
1988
Working Days
Monday To Friday
Related Products
Explore Related Categories
More Product From This seller
Seller Details
Beijing, Beijing
Sales Manager
Mr Steven Tan
Address
Floor 2 & 3 Building 3 No.27 Yongwang Road, Daxing District, Beijing, Beijing, 102600, China
other products in Beijing
Report incorrect details