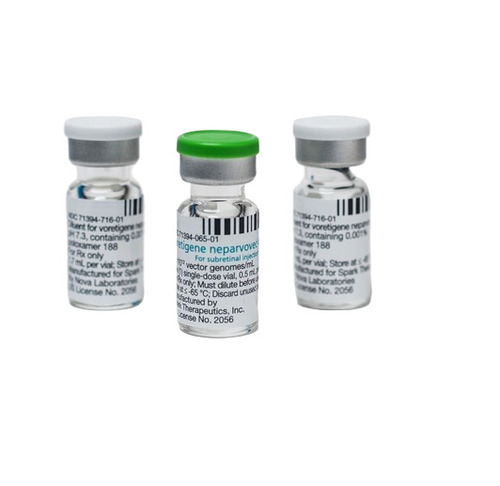
Luxturna Injection - Origin: India
Price: 45700.00 INR / Box
(45700.00 INR + 0% GST)
Get Latest Price
Minimum Pack Size :
1
In Stock
Product Specifications
| Indication | to treat vision loss caused by inherited retinal dystrophy |
| Origin | india |
| Dosage Form | injection |
| Fermentation Smell | Normal Smell |
| Storage Instructions | Cool and Dry Place |
| Shelf Life | 3 Years |
| Payment Terms | Paypal, Cash Advance (CA), Western Union, Others |
| Supply Ability | 100 Per Week |
| Delivery Time | 6 Days |
| Sample Available | Yes |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | Box |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa |
| Main Domestic Market | All India |
| Product Unit | 1 Piece/Pieces |
| Mop | 1 |
| Currency | INR |
| Stock Quantity | 5 |
| Returnable | No |
| Moq | 1 |
| Price Type | fixed |
| Brand Name | luxturna |
| Unit Type | Piece/Pieces |
| Price | 45700.00 INR (Approx.) |
| Minimum Ordered Packs | 1 |
| Minimum Order Quantity | 1 |
| GSTIN | 0% |
Product Overview
Key Features
Luxturna (voretigene neparvovec-rzyl) is a gene therapy used to treat inherited retinal dystrophy (IRD) caused by mutations in the RPE65 gene. It is specifically indicated for patients with confirmed biallelic RPE65 mutations and sufficient viable retinal cells. Luxturna helps restore vision by delivering a functional copy of the RPE65 gene to retinal cells, allowing them to produce the necessary protein for the visual cycle.
Luxturna Injection is a gene therapy product designed to treat inherited retinal dystrophy caused by biallelic mutations in the RPE65 gene.
Key Features:-
Gene Therapy: Delivers a functional RPE65 gene to retinal cells (, ).
Administration: Given as a single subretinal injection in each eye by an experienced ophthalmologist (, web:. Dose: Contains 1.5 x 10^11 vector genomes per eye in a 0.3 mL volume after dilution (, web:. Delivery Method: Administered via subretinal injection after vitrectomy (, web5. Purpose: Aims to restore visual cycle function by replacing defective gene ().
Treatment Setting: Performed at specialized, trained treatment centers (web7. Storage: Supplied as a sterile concentrate needing thawing and dilution before use (, ).
Rare Disease Use: Approved for rare inherited retinal diseases, including retinitis pigmentosa and Lebera s congenital amaurosis (web9. Safety: Requires immunosuppressant pre-treatment to reduce rejection risk ().
Limitations: Effective only if sufficient viable retinal cells remain (, web:This product represents a significant advancement in treating specific genetic eye disorders through gene therapy.
Company Details
Welcome to Hosmat Hospital Private Limited As a trusted wholesaler of pharmaceutical medicines and chemicals, we prioritize the health and well-being of our customers. Our extensive range of products is sourced from reputable manufacturers and is designed to meet the diverse needs of healthcare professionals and institutions.
Business Type
Distributor, Supplier
Employee Count
10
Establishment
2017
Working Days
Monday To Sunday
GST NO
29AABCP2221Q1Z6
Payment Mode
Paypal
Certification
GST 29AABCP2221Q1Z6
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 29AABCP2221Q1Z6
Bengaluru, Karnataka
Sales Manager
Mr Satwinder Gupta Singh
Address
Hosmat Hospital No. 45, Magrath Road, Off Richmond Road, Bengaluru, Karnataka, 560025, India
anti cancer injection in Bengaluru
Report incorrect details