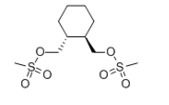
Lurasidone Intermediate - Pharmaceutical Grade, 99% Purity White Powder | Fda Approved For Schizophrenia And Bipolar Disorders
Price:
Get Latest Price
In Stock
Product Specifications
| Molecular Formula | C10H20O6S2 |
| Molecular Weight | 300.394 Grams (g) |
| Place of Origin | China |
| Other Names | (R,R)-1,2-bis(Methan esulfonyloxyMethyl) cyclohexane |
| CAS No | 186204-35-3 |
| Type | Other |
| Grade | Medicine Grade |
| Usage | Lurasidone has been approved for treatment of schizophrenia by the FDA since 2010. In the USA since 2013, it is also approved for the treatment of depressive episodes associated with bipolar I disorder as well as bipolar II disorder in adults when used alone or in combination with lithium, valproate, or lamotrigine. |
| Purity | 99%min |
| Appearance | white powder |
| Application | Pharmaceutical Industry |
| Form | Powder |
| FOB Port | Shanghai |
| Payment Terms | Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T) |
| Supply Ability | 500kg Per Day |
| Delivery Time | ship immediately after confirmation if there have material in stock Days |
| Sample Available | Yes |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | foil/bag/bottle |
| Main Export Market(s) | Australia, North America, Eastern Europe, Western Europe, Africa, Central America, Middle East, South America, Asia |
Product Overview
Key Features
Company Details
Business Type
Exporter, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
250
Establishment
2004
Working Days
Monday To Sunday
Related Products
Explore Related Categories
More Product From This seller
Seller Details
Shanghai, Shanghai
Ceo
Mr. Peter Dong Wang
Members since
13 Years
Address
Suite B-10, Chuansha Road 6999#, Shanghai, Shanghai, 201203, China
pharmaceutical additives in Shanghai
Report incorrect details




















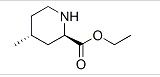

![Ethyl N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-Formyl-1-Methyl-3-Oxododecahydronaphtho[2,3-c]Furan-6-yl]Carbamate - CAS 900180-06-5, 99% Purity White Solid for Pharmaceutical Use](https://tiimg.tistatic.com/fp/1/003/895/ethyl-n-1r-3ar-4ar-6r-8ar-9s-9as-9--939.jpg)