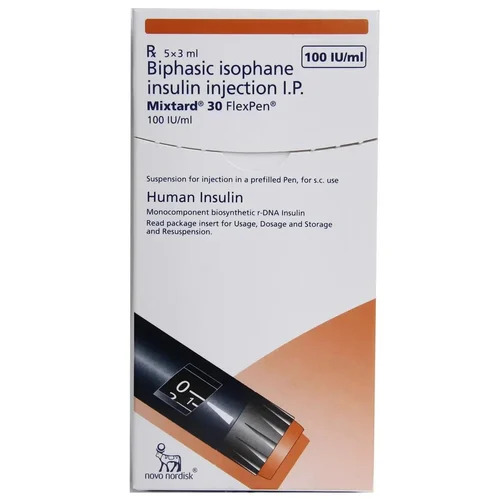
Admelog Rapid Acting Insulin Lispro Injection
Price Trend: 39.00 - 99.00 USD / Box
Get Latest Price
Minimum Order Quantity :
1 Box
In Stock
Product Specifications
| Dosage Form | Injectable |
| Physical Form | Liquid |
| Dosage | As directed by physician |
| Dosage Guidelines | As directed by physician |
| FOB Port | USA |
| Payment Terms | Paypal, Cash Against Delivery (CAD), Cash on Delivery (COD), Cash Advance (CA), Cash in Advance (CID), Cheque, Days after Acceptance (DA), Delivery Point (DP), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Letter of Credit (L/C) |
| Supply Ability | 1000000 Per Week |
| Delivery Time | 15 Days |
| Sample Available | Yes |
| Sample Policy | Free samples are available |
| Packaging Details | Box |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa |
| Main Domestic Market | All India |
Product Overview
Key Features
ADMELOG is a rapid-acting human insulin analog indicated to improve glycemic control in adults with type 2 diabetes and adults and children (3 years and older) with type 1 diabetes.
Important Safety Information
CONTRAINDICATIONS
ADMELOG is contraindicated during episodes of hypoglycemia, and in patients with hypersensitivity to insulin lispro or to any of its excipients.
WARNINGS AND PRECAUTIONS
Insulin pens and needles must never be shared between patients, even if the needle is changed. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Hypoglycemia is the most common adverse reaction associated with insulins, including ADMELOG, and may be life-threatening.
Accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. To avoid medication errors between ADMELOG and other insulins, instruct patients to always check the insulin label before each injection.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including ADMELOG. If hypersensitivity reactions occur, discontinue ADMELOG, treat per standard of care and monitor until symptoms and signs resolve.
All insulin products, including ADMELOG, can cause hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia.
Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs) with insulin. Observe for signs and symptoms of heart failure. Consider dosage reduction or discontinuation of TZD if heart failure occurs.
ADVERSE REACTIONS
Adverse reactions associated with ADMELOG include hypoglycemia, hypokalemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and peripheral edema.
DRUG INTERACTIONS
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (eg, beta-blockers, clonidine, guanethidine, and reserpine).
IMPORTANT SAFETY INFORMATION FOR ADMELOG (INSULIN LISPRO INJECTION) SOLOSTAR
ADMELOG SoloStar is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow the steps in the Instruction Leaflet accompanying the pen; otherwise they may not get the correct amount of insulin, which may affect their blood glucose levels.
IMPORTANT SAFETY INFORMATION FOR ADMELOG (INSULIN LISPRO INJECTION) USE IN PUMP
Pump failure or insulin infusion set, or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause is necessary. Interim subcutaneous injections with ADMELOG may be required. Patients using a pump must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Company Details
Business Type
Exporter, Importer, Manufacturer, Service Provider, Distributor, Supplier, Trading Company, Wholesaler, Retailer, Dealer, Fabricator, Producer
Employee Count
50
Establishment
2017
Working Days
Monday To Sunday
Related Products
Explore Related Categories
More Product From This seller
Seller Details
California, Missouri
Export Manager
Mr Timothy Florian
Address
28955, Avenue Sherman Valencia, California, Missouri, 91355, United States
insulin pen in California
Report incorrect details