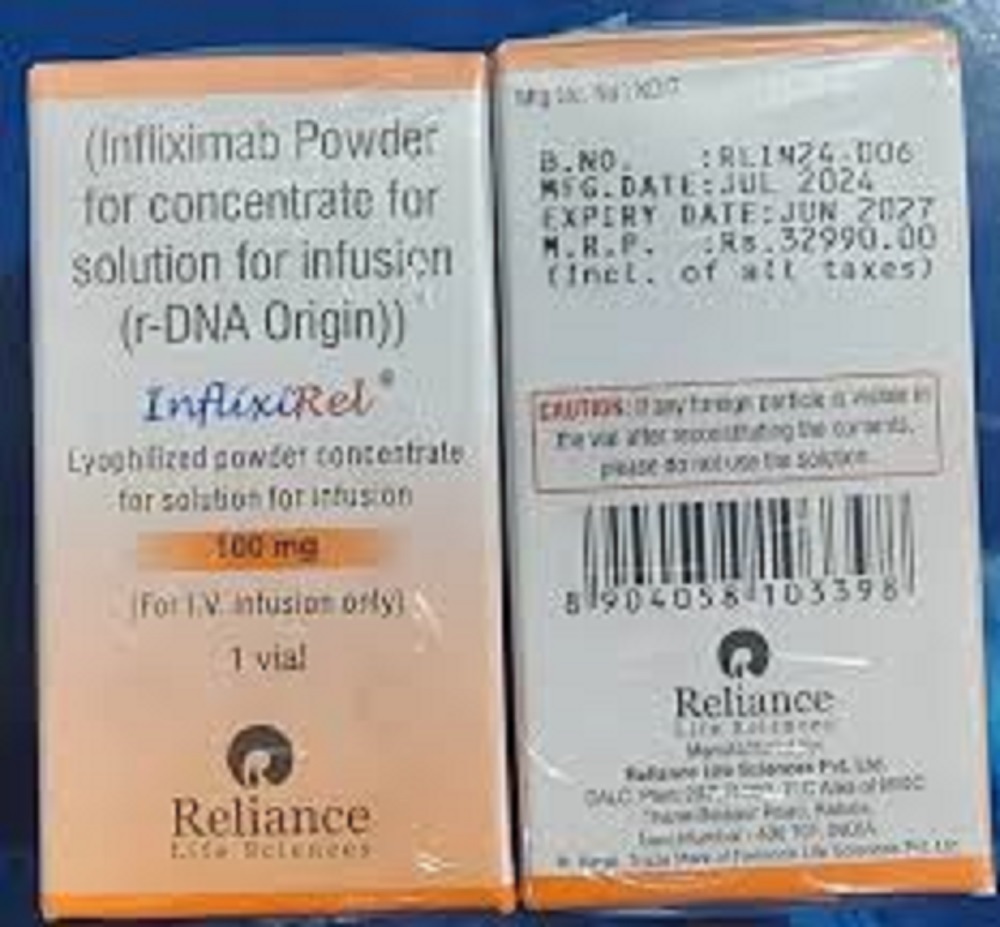
Inflixirel 100 Mg Injection
Price: 10500 INR / Vial
Get Latest Price
Minimum Order Quantity :
1 Vial
Brand Name :
Inflixirel 100 Mg Injection
In Stock
Product Specifications
| Salt Composition | Infliximab 100 mg |
| Pacakaging (Quantity Per Box) | 1 vial per box |
| Indication | Autoimmune and inflammatory disorders such as rheumatoid arthritis, Crohns disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis |
| Origin of Medicine | Biological |
| Dosage Form | Lyophilized Powder for Solution for Intravenous Infusion |
| Drug Type | Other, Prescription Biological Drug |
| Ingredients | Infliximab |
| Physical Form | Other, Injection |
| Function | Other, Immunosuppressive/Anti-TNF (tumor necrosis factor) agent |
| Recommended For | Adults and pediatric patients with rheumatoid arthritis, CrohnaEURtms disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis |
| Dosage | As directed by the physician |
| Dosage Guidelines | To be administered intravenously under medical supervision |
| Suitable For | Other, Adults and children (as advised by doctor) |
| Quantity | 100 mg per vial |
| Storage Instructions | Store in a refrigerator (2AdegC - 8AdegC). Do not freeze. |
Company Details
Focusing on a customer-centric approach, DEV MEDICAL has a pan-India presence and caters to a huge consumer base throughout the country. Buy Common Medicines & Drugs in bulk from DEV MEDICAL at Trade India quality-assured products.
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company, Wholesaler, Retailer, Dealer
Employee Count
50
Establishment
2020
Working Days
Monday To Sunday
GST NO
27ANQPV9165Q2ZH
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 27ANQPV9165Q2ZH
Pune, Maharashtra
Proprietor
Mr Dev
Members since
4 Years
Address
Shop No - 6, Survey Number - 32, Kamal Heights, Aditya Birla Hospital Marg, Datta Nagar, Thergaon, Pune, Maharashtra, 411033, India
infliximab injection in Pune
Report incorrect details