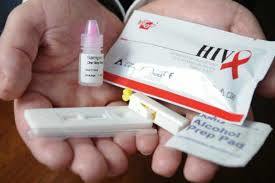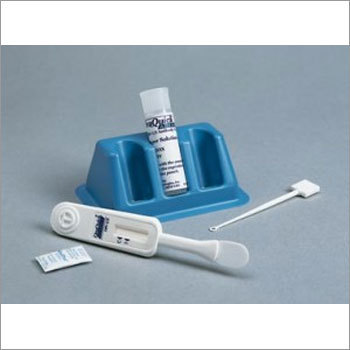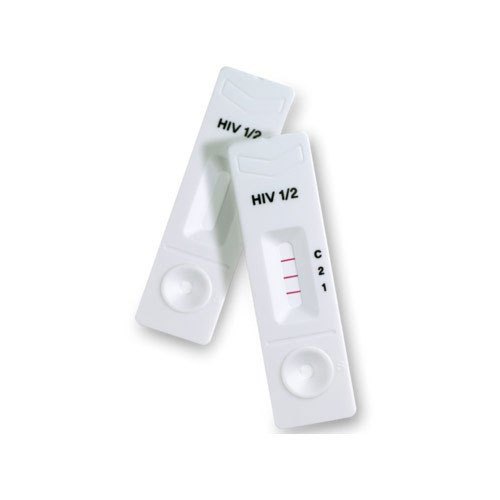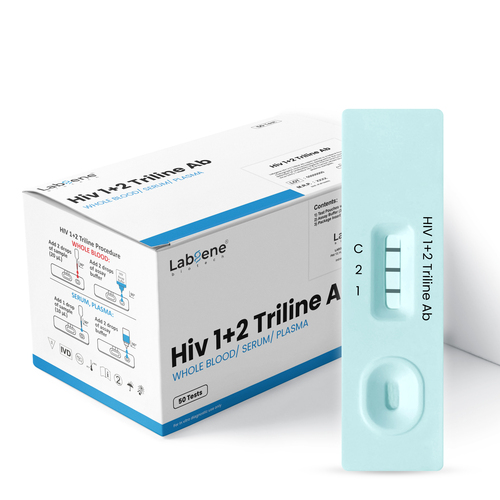
Labgene Hiv 1+2 Triline Rapid Test Kit - Manual, Plastic, Lateral Flow Immunoassay | 100% Sensitivity, Fast 15-minute Results, Easy To Use
Price: 14.70 INR / Kit
(14.00 INR + 5% GST)
Get Latest Price
MRP:
14.70 INR / Kit
Weight :
16.00 Gram
1 Pack Contains :
25
Minimum Pack Size :
40
In Stock
Product Specifications
| Application | Hospital, Diagnostic Centre |
| Condition | New |
| Features | Easy-to-use, no special equipment required |
| Storage Instructions | 2 Degree Celsius to 30 Degree Celsius |
| Usage Type | Professional In-Vitro Diagnostic (IVD) Use Only |
| Shelf Life | 24 Months |
| Accuracy | 100 % |
| Equipment Type | Labgene HIV 1+2 Triline Rapid Test Kit |
| Material | Plastic |
| Technology | Lateral flow immunoassay |
| Portable | Yes |
| Wall Mounted | No |
| Operating Type | Manual |
| Use | Detection of Antibody for HIV 1+2 Antibody |
| Power Source | Manual |
| Weight | 180 Grams (g) |
| FOB Port | Ahmedabad & Mumbai |
| Payment Terms | Cheque, Others, Cash on Delivery (COD), Cash in Advance (CID) |
| Supply Ability | 500000 Per Month |
| Delivery Time | 1 Week |
| Sample Available | No |
| Sample Policy | If order is confirmed we will reimburse the sample cost |
| Packaging Details | Carton Box |
| Main Export Market(s) | Asia, Africa, South America |
| Main Domestic Market | All India |
| Certifications | ISO 13485:2016, NIB evaluated, ISO 90001, CE, MDR:2017 |
| Mop | 40 |
| Price | 14.00 INR (Approx.) |
| Shipping Type | free |
| Pkg Box Breadth | 12.20 cm |
| Weight | 16.00 Gram |
| Pkg Box Length | 13.50 cm |
| Returnable | No |
| Unit Type | Kit/Kits |
| Product Unit | 40 Kit/Kits |
| Currency | INR |
| Stock Quantity | 100000 |
| MRP | 14.70 INR |
| Pkg Box Height | 7.00 cm |
| Packsize | 25 |
| Brand Name | Labgene |
| Price Type | fixed |
| GSTIN | 5% |
| Minimum Ordered Packs | 40 |
Product Overview
Key Features
Sample Type : Blood
Number of Reactions(Preps)/Kit : 50 Kits
Brand : Labgene
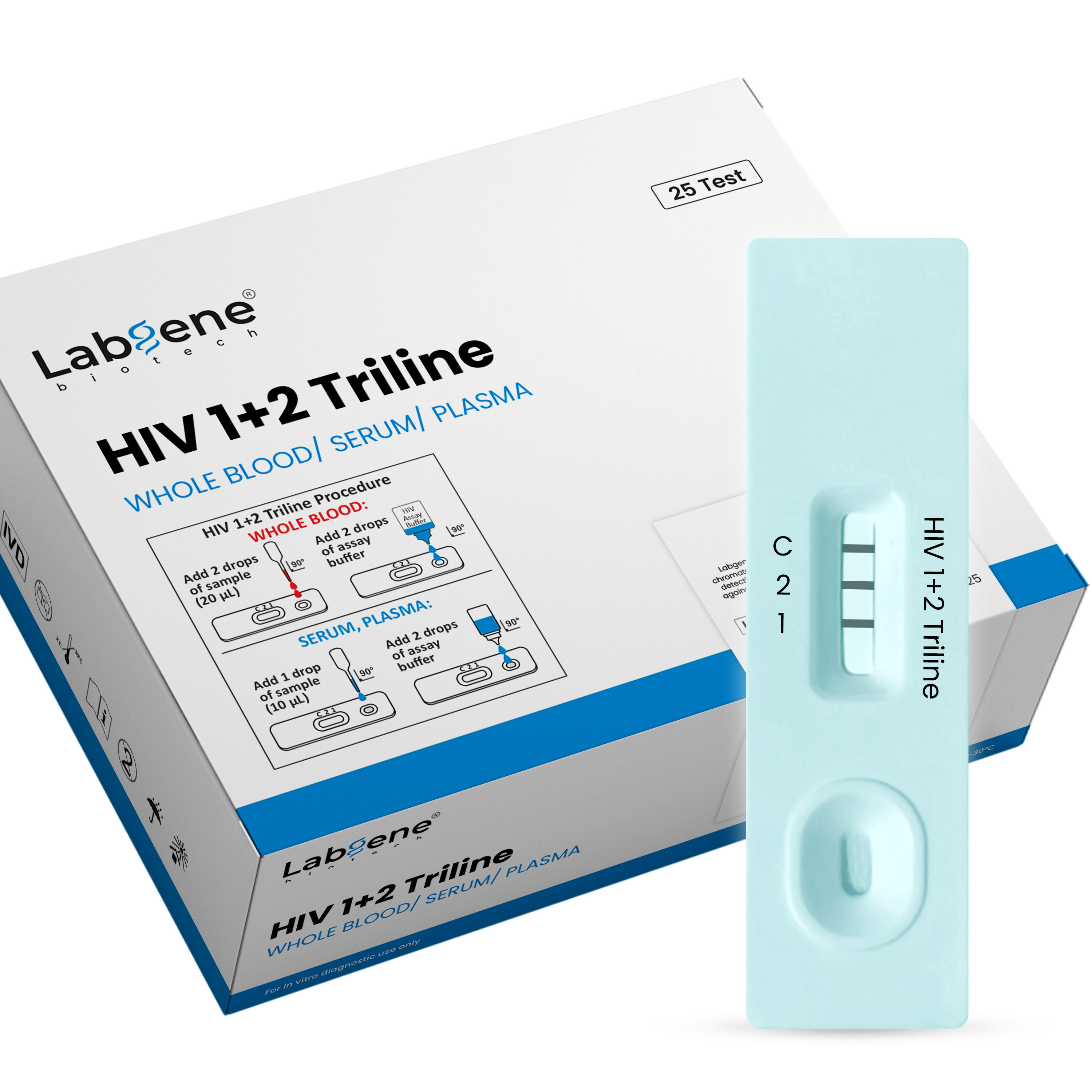
Accurate. Reliable. Fast HIV Detection in Minutes
The Labgene HIV 1+2 Triline Ab Rapid Test Kit is a high-performance lateral flow immunoassay designed for the qualitative detection and differentiation of antibodies to HIV-1 and HIV-2 in human whole blood, serum, or plasma samples.
With 100% sensitivity and 100% specificity, this CE-certified rapid test delivers fast, reliable, and easy-to-read results within 20 minutes, making it an essential tool for professional diagnostic use in laboratories, clinics, and field screening programs.
Key Features
Dual-line detection for HIV-1 and HIV-2 antibodies
Results in 15 minutes quick and efficient testing
Works with whole blood, serum, or plasma samples
Easy-to-use with dropper and buffer provided
Room temperature storage (2C to 30C)
Evaluated by National Institute of Biologicals (India)
High correlation with ELISA & other rapid tests
How It Works
The test detects antibodies targeting gp41 and gp120 antigens (for HIV-1) and gp36 antigen (for HIV-2). When the sample reacts with these coated antigens, distinct red-magenta lines appear in their respective areas if antibodies are present. An internal control line ensures test validity.
Kit Contents
Individually pouched test devices
Assay buffer vials
Disposable droppers
Silica gel for moisture control
Alcohol swabs and sterile lancets (in select packs)
Product insert (IFU)
Available Pack Sizes: 25Tests & 50Tests
(Custom packaging with lancets and swabs also available)
Frequently Asked Questions (FAQs)
Who should use this test?
This test is intended for professional in vitro diagnostic use. It is ideal for use in clinical laboratories, hospitals, blood donation camps, and diagnostic centers.
What types of samples can be used?
The test works with whole blood, serum, or plasma. It supports both venipuncture and finger-prick blood collection.
How accurate is the Labgene HIV 1+2 Rapid Test?
The test has demonstrated 100% sensitivity and 100% specificity during internal and external evaluations, including by the National Institute of Biologicals (NIB).
How do I interpret the results?
Two Lines (C + HIV-1 or C + HIV-2): Positive
Three Lines (C + HIV-1 + HIV-2): Positive for both
One Line (C only): Negative
No Control Line (C): Invalid, repeat with new test
What if my result is positive?
A positive result is preliminary and should be confirmed with a Western Blot or other confirmatory tests in accordance with national HIV testing guidelines.
How should I store the test kits?
Store the kits in a sealed pouch between 2C and 30C. Do not freeze. Use the test before the expiration date indicated.
Can I reuse the test?
No. The test is single-use only and should be safely discarded after use as per biomedical waste protocols.
Is this test WHO or FDA approved?
The test has been validated by leading national bodies such as the NIB (India) and is manufactured in an ISO-certified facility. Please refer to local regulatory approval requirements for use in your region.
Company Details
Labgene is an emerging biotechnology company, the only one which produces multiple diagnostics kits under a single roof. Our dedicated team of highly experienced professionals work hard to produce high-quality diagnostics kits for the world.
Business Type
Exporter, Manufacturer, Service Provider, Distributor, Supplier, Trading Company, Wholesaler, Dealer, Producer
Employee Count
80
Establishment
2019
Working Days
Monday To Saturday
GST NO
24AADCL8897L1ZQ
Payment Mode
Cash in Advance (CID)
Certification
ISO 13485:2016, ICMR,NIB evaluated, ISO 90001, CE, WHO,FDA
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AADCL8897L1ZQ
Ahmedabad, Gujarat
Sales Manager
Ms Falguni Ji
Members since
1 Years
Address
Gf, Plot No 13, 14, Kamla Amrut Inditech Park, Chhatral Kadi Road, Indrad, Ahmedabad, Gujarat, 382715, India
hiv test kit in Ahmedabad
Report incorrect details