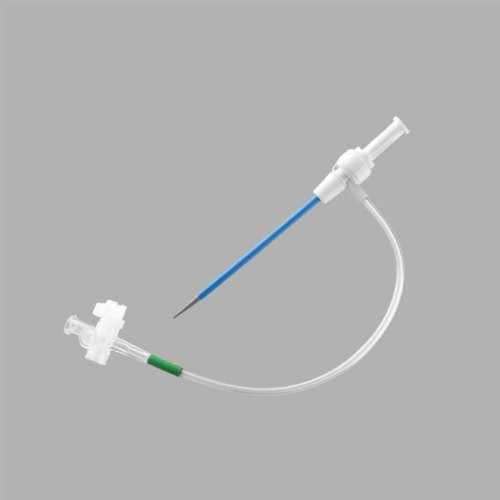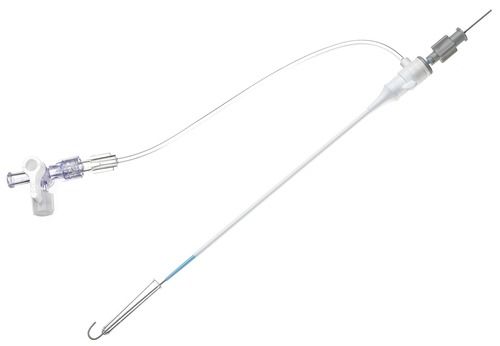
High Grade Shoocin Introducer Kit Application: Industrial
Price: 650 INR
Get Latest Price
Minimum Order Quantity :
20
In Stock
Product Specifications
| Material | Polyurethane |
| Type | Introducer Kit |
| Sheath type | Radial/Femoral |
| Guidewire material | Stainless Steel |
| Needle gauge | 20G |
| Usage | Vascular access procedures in radial, femoral, and long sheath applications. |
| Sterility | Sterile |
| Features | Smooth access, Tissue protection, Easy insertion, Customizable kit, Advanced material |
| FOB Port | NEW DELHI |
| Payment Terms | Telegraphic Transfer (T/T) |
| Supply Ability | 300000 Per Month |
| Delivery Time | 1 Week |
| Sample Available | Yes |
| Sample Policy | Sample costs shipping and taxes has to be paid by the buyer |
| Main Domestic Market | All India |
| Certifications | CE FDA |
Product Overview
Key Features
Advanced material improves bendability and flexibility without gapping or kinking.
Quarter-turn-screw dilator locking system ensures the smooth transition from dilator to sheath, and prevents the pull out of the dilator because of the friction during insertion.
Minimized possibility of damaging tissue upon sheath entry, advancement and withdrawal, providing smoother and easier vascular access even through difficult access sites.
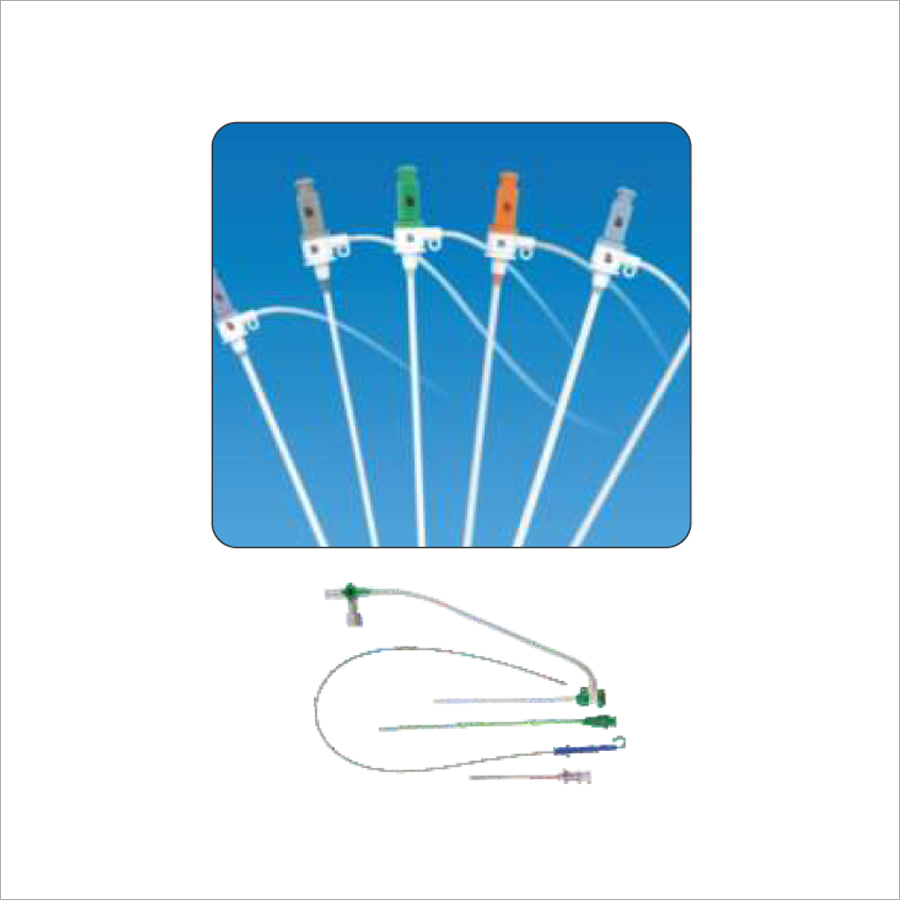
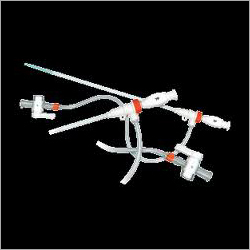
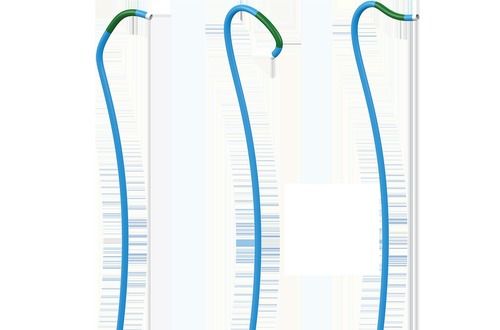
Including types of Standard, Radial, Femoral. Long Sheaths.
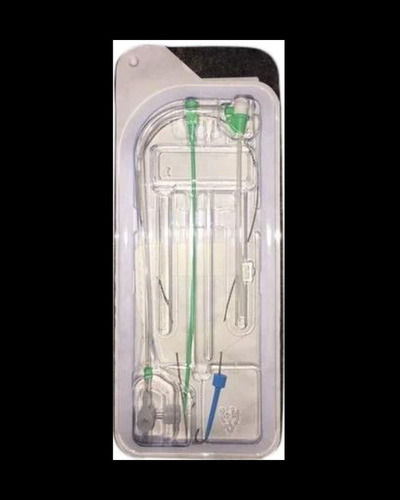
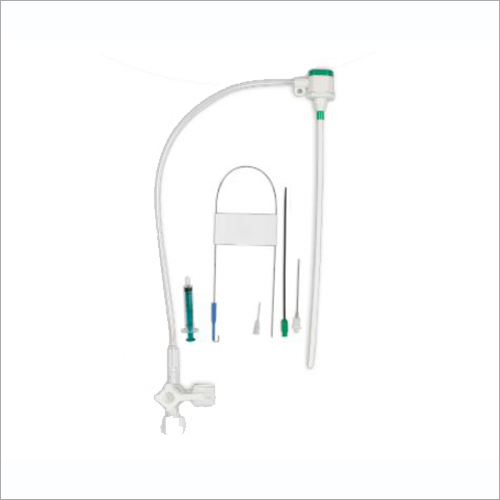
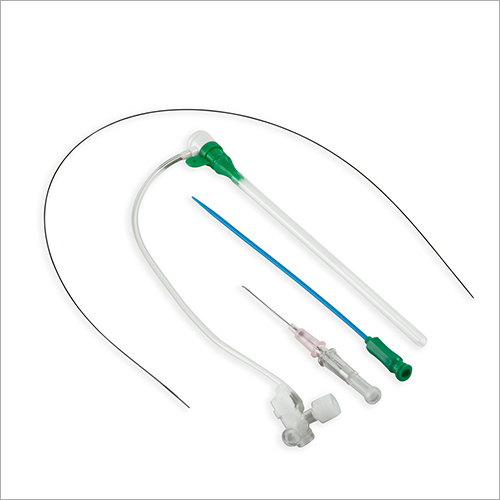
Full kit Components:
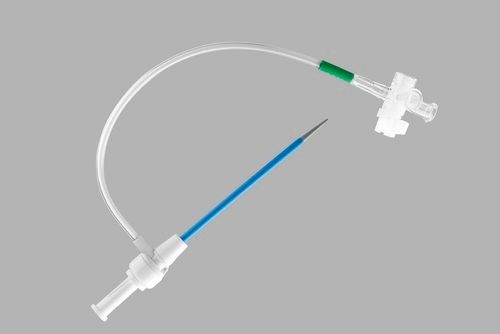
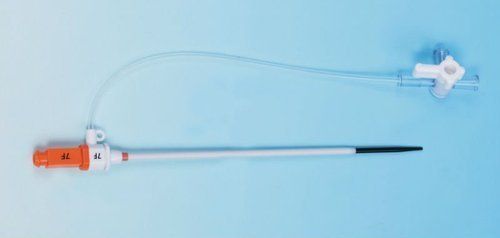
Introducer Sheath (radial / femoral / long sheath) (1)
Stainless Steel Guide Wire (straight / J angled/ 135A angled) (1)
Introducer Needle (1)
Scalpel (1)
Syringe (1)
The Introducer Kit can be customized by:
a)Remove the every single component includes guide wire, introducer needle (IN), scalpel (K) and syringe (S).
b)Replace the stainless steel guide wire (straight SS/J SJ/135A SW) into Nitinol guide wire (straight NS/J NJ/135A NW) or hydrophilic guide wire (straight HS/135A HW).
c)Replace the introducer needle (IN) into intravascular catheter (IC).
(e.g. Radial sheath/6F/7cm, 0.021" straight stainless steel guide wire/45cm, 20G introducer needle, without scalpel and syringe: RS060721-SS45-IN20)
Please contact for detailed catalogue numbers of customized kits.
ORDERING INFORMATION
Please contact local distributors for detailed information.
Company Details
Business Type
Manufacturer, Supplier, Trading Company, Producer
Employee Count
6331
Establishment
1999
Working Days
Monday To Sunday
GST NO
07AADCL0623N1ZI
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AADCL0623N1ZI
New Delhi, Delhi
Country Manager
Mr. Vivek Jhawar
Address
DSM-117, First Floor, DLF Towers, 15, Shivaji Marg, Najafgarh Road, New Delhi, Delhi, 110015, India
Report incorrect details