- Tradeindia
- Common Medicines & Drugs
- Probiotic Sachets
Probiotic Sachets
(289 products)Powder Cholecalciferol Sachets
Price: 100 INR/Piece
MOQ1000 Box/Boxes
Physical FormPowder
DosageAs per the doctor's advice
Dosage GuidelinesAs per the doctor's advice
Suitable ForSuitable For All
Storage InstructionsKeep at cool and Dry Place
Alax Bioresearch Private Limited
Ahmedabad
 Super Bonanza
Super Bonanza Super Premium
Super Premium Premium Seller
Premium Seller6 Years
 Super Premium
Super PremiumPrebiotic Supplement
Price: 800 INR/Box
MOQ3000 Box/Boxes
FOB PortMUMBAI
Payment TermsCash in Advance (CID)
Supply Ability10000 Per Day
View More

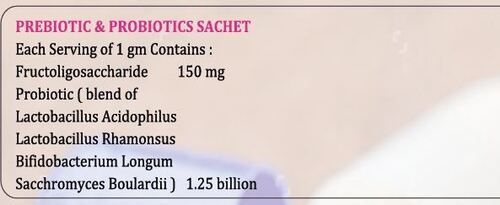
Pre & Probiotic Sachet Dosage Form: Powder
Price : 300 INR
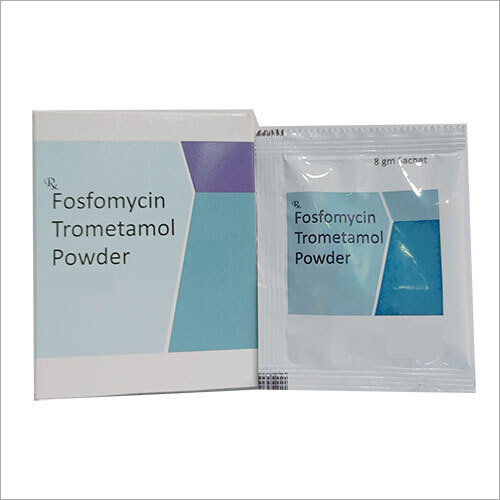
Fosfomycin Trometamol Powder Generic Drugs
Price Trend: 115.00 - 130.00 INR/Pack
MOQ3000 Pack/Packs
Drug TypeGeneric Drugs
IngredientsFosfomycin Trometamol Powder
Physical FormPowder
Recommended ForRecommended For
Dosage GuidelinesDosage Guidelines
Quantity3000 Pieces
Pentasa Mesalazine 1G Recommended For: To Treat Mild To Moderate Ulcerative Colitis
Price: 2800 INR/Box
MOQ100 Box/Boxes
Recommended ForTo treat mild to moderate ulcerative colitis
Dosage GuidelinesAs Per Instructions
Storage InstructionsKeep Dry Place
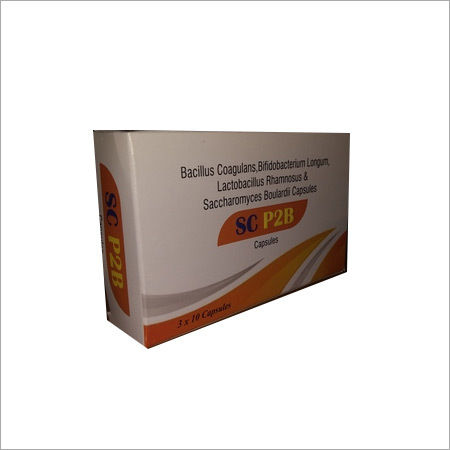
Bacillus Coagulans & Probiotic Blend Capsules - Nutritional Support for Energy | 2-Year Shelf Life, Store in Cool Place, With Water Recommendations
Price Trend: 1.00 - 5.00 USD ($)/Box
MOQ5000 Box/Boxes
EfficacyPromote Nutrition
Shelf Life2 Years
IngredientsBacillus Coagulans,Bifidobacterium Longum,Lactobacillus Rhamnosus & Saccharomyces Boulardii Capsules
Sub IngredientsBacillus Coagulans,Bifidobacterium Longum,Lactobacillus Rhamnosus & Saccharomyces Boulardii Capsules
FunctionProvide Energy
Dosage GuidelinesWith Water
View More

Pre and Probiotic Sachets
Price Trend : 1.00 - 5.00 USD ($)
Entroflora Capsule General Medicines
Price: 197 INR/Piece
MOQ10-1000 Piece/Pieces
Drug TypeGeneral Medicines
IngredientsProbiotics
Physical FormTablets
FunctionSimplifies Digestion
Dosage GuidelinesAs Per Doctors prescription
Suitable ForWomen, Adults, Suitable For All
Pre and Probiotic Sachet
Price: 3 INR/Box
MOQ1000 Box/Boxes
Product DescriptionPre and Probiotic Sachet
View More
Pre & Prebiotic And FOS Sachets
Get Best Deal
Cholecalciferol - 60,000IU in 10x1gm Sachets | General Medicine, Suitable for All, Store in Cool Dry Place, Dosage as per Doctor's Advice
Price: 250 INR/Box
MOQ500 Box/Boxes
Drug TypeGeneral Medicines
Physical FormOther, Sachet
DosageAs per doctor advice
Suitable ForSuitable For All
Storage InstructionsCool and dry place
Lactobacillus Sporogenes Probiotics - Storage Instructions: Keep At Cool And Dry Place
Price: 450 INR/Kilograms
MOQ25 Kilograms/Kilograms
Drug TypeOther Types
Physical FormOther, Capsule, Powder
Dosage100 mg
Storage InstructionsKeep at cool and Dry Place
View More

Powder Prebiotic And Probiotic Sachet
Price : 12 INR
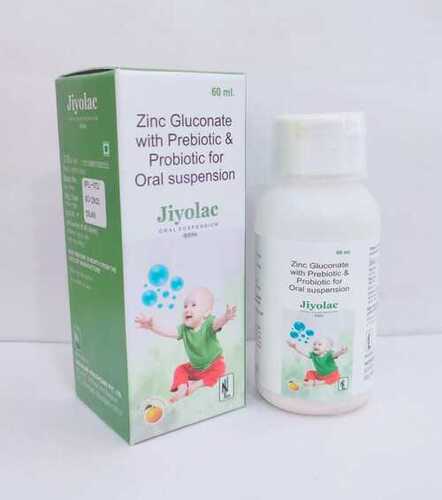
60ml Prebiotic And Probiotic With Zinc Oral Suspension
Price: 40 INR/Bottle
MOQ100 Bottle/Bottles
Origin of MedicineSynthetic
Salt CompositionZinc Gluconate Lactobacillus Saccharomyces boulardii (or other relevant strains)
Pacakaging (Quantity Per Box)1 bottle per box
Drug TypeOther, Prebiotic and Probiotic with Zinc
IngredientsPrebiotics Probiotics Zinc
Physical FormOther, Oral Suspension
Probiotic Sachets - Convenient Single-Serving Packets | Enhances Gut Health, Supports Digestion
MOQ10 Pack/Packs
Physical FormOther
Bio-Life Sachet Specific Drug
Price: 1000 INR/Box
MOQ10000 Box/Boxes
Drug TypeSpecific Drug
Physical FormPowder
DosageAs per the doctor's advice
Dosage GuidelinesAs per the doctor's advice
Storage InstructionsKeep at cool and Dry Place
Top Rated Products
Pentasa Mesalazine 1G Recommended For: To Treat Mild To Moderate Ulcerative Colitis
Dheer Healthcare Private Limited
FAQs Related to Probiotic Sachets
- You suffer from asthma and allergic rhinitis.
- Food poisoning has struck you.
- You seem always ill.
- You have a mood problem (or several).
- You're on antibiotics now
- Skin problems like acne and psoriasis plague you.
Pre & Probiotic Sachet With Zinc - 1 GM Powder, Balanced Micro Flora Support & Gut Health Booster
Drug TypeDrug Solutions
Physical FormPowder
View More

Pre Probiotic Sachet with Zinc
Price : 150 INR
Prebiotic & Probiotic Sachet Dosage Form: Powder
Price: 2.78 USD ($)/Box
MOQAs required by client. Box/Boxes
OriginIndia
EfficacyPromote Nutrition
Drug TypeHealth Supplements
IngredientsPrebiotic & Probiotic
Physical FormPowder
FunctionOther
Prebiotic + Probiotic with Zinc Sachet
Price: 0.20 USD ($)/Number
MOQ1000000 Number
FOB PortAdani port Mundra /Hajira Adani /Nhava-Sheva JNPT
Supply Ability29000 Per Day
Delivery Time29 Days
10 Kg Matsya Mitra Soil Probiotics - Application: Agriculture
Price: 900 INR/Pack
MOQ1 Pack/Packs
Physical StatePowder
Release TypeControlled
ApplicationAgriculture
ColorBrown
StorageDry Place
Sachets - Custom Powdered Solutions | General Medicines with Pre-Biotic, Probiotic, Cholecalciferol, L-Arginine
Price: 55 INR/Bag
MOQ10000 sachets Bag/Bags
Drug TypeGeneral Medicines
Ingredientspre-biotic, probiotic, cholecalciferol, l-arginine, etc
Formulations TypeOther
Physical FormPowder
Formulations FormOther
Storage InstructionsOther
PRE AND PROBIOTIC SACHETS
Price: 1 INR/Bundle
MOQ1000 Bundle
Main Domestic MarketWest India
Prebiotic & Probiotic Capsules
Price: 25060 INR/Piece
MOQ5000 Piece/Pieces
Supply Ability3000 Per Month
Delivery Time1 Days
Main Domestic MarketAll India
Powder For Constipation - 1 g x 20 Sachets | Probiotic Blend with Lactobacillus, Bifidobacterium & Saccharomyces for Digestive Health
MOQ5000 Box/Boxes
OriginIndia
Shelf Life24 Months
EffectHelps reduce gas flatulence/ Strengthen immunity & improve digestion
IngredientsLactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, Bacillus coagulans, Saccharomyces boulardii
DosageEach serving is 1 g, take serving(s) as directed by Dietician/ Physician
Dosage FormPowder
View More

Hitic Sachet
Get Best Deal

Hitic Plus Sachet
Get Best Deal

Probiotic Drink Sachets
Get Best Deal

Saccharomyces Boulardii Sachet
Get Best Deal
Pre Probiotic Sachet - Dosage Form: Tablet
Price: 170 INR/Pack
MOQ500 Pack/Packs
OriginIndia
Shelf Life6 Months
DosageAs Per Physician
Dosage GuidelinesWith Warm Water
Dosage FormTablet
Storage InstructionsCool & Dry place
Lactobacillus Acidophilus Additives: Food
Price: /Kilograms
MOQ10 Kilograms/Kilograms
AdditivesFood
GradeFood
Product NameLactobacillus acidophillus
Product TypeProbiotic
FormPowder
TasteTastless
Prebiotic Strains Probiotic Fos Zinc Sachets - Drug Type: General Medicines
Price: 350 INR/Box
MOQ10 Box/Boxes
Origin of MedicineIndia
Drug TypeGeneral Medicines
Physical FormPowder
Dosage1.5 gm
Dosage GuidelinesAs Suggested
Storage InstructionsCool & Dry Place
Rhodocom Plus - Liquid Multi-Strain Probiotic Supplement, Optimized for Aquatic Health & Nutrient Absorption, Enhances Water Quality and Prevents Sludge
Price: 90 INR/Pack
MOQ1 Pack/Packs
Suitable ForAquatic Animals
ApplicationWater
FunctionImmune System, Heathycare supplement, Nutritional Medicine, Promote Digestion
EfficacyFeed Preservatives, Promote Healthy, Promote Growth
Disease preventionAnti-Fatigue, Improve immunity
Prebiotic And Probiotic Sachets - Ingredients: Dietary Supplements
Price: 30 INR/Box
MOQ500 Box/Boxes
Shelf Life18 Months
Purity100%
IngredientsDietary Supplements
DosageDaily
Dosage GuidelinesWith Water
Storage InstructionsCool Dry Place
Prebiotics And Probiotic Sachet - Dosage Form: Tablet
Price: 380 INR/Box
MOQ300 Box/Boxes
Dosage FormTablet
IngredientsAcotiamide 100 mg
Physical FormTablets
Recommended ForHospital
Dosage GuidelinesUse as directed by a healthcare professional
Storage InstructionsStore in a cool
Pre Pro Biotic Plus Sachet
Price: 20 INR/Piece
MOQ1000 Piece/Pieces
Dosage FormPowder
Physical FormPowder
DosageAs prescribed by physician
Dosage GuidelinesAs per physician advice
Storage InstructionsCool and dry place
Latest from Probiotic Sachets
Priotic Prebiotic And Probiotic Sachet, 1x20 Pack
By:
Zenon Healthcare Ltd.
Probiotic Sachet
By:
Prosafe Biologicals
Prebiotic Probiotic Sachet
By:
Nuchem Organics
Ecotic Sachets
By:
Eco Life Care Pvt. Ltd.
Pre and Probiotic Sachets
By:
Lexicare Pharma Pvt. Ltd.
What is Probiotic Sachet?
What is Probiotic Sachet used For?
What are Benefits of Probiotics?
Probiotic Sachet Dosage
Client Testimonials & Reviews

DevendraMathuria
HEET HEALTHCARE PVT. LTD.
Tradeindia has been a true game-changer for us. Now the reach of our organization has spanned all the geographical boundaries. Now we are able to showcase our products much more efficiently and the number of inquires have also increased tremendously. Kudos to the Tradeindia team.

Tradeindia has been doing great work in B2B segment, its so much easy to reach potential buyers through their portal and App. Their service is good and quick revert from their back-end team. Suggest others to avail their services to improve business.

SandeepGokhru
Luckys Pharma Lab Pvt Ltd
We are working with tradeindia form last 4 years. Tradeindia helps us to make our presence on top search engines and also provides us good numbers of business leads and inquiries, which helps us to grow our sales.

UjjawalKumar
R B REMEDIES PVT. LTD.
I am very satisfied with GetDistributors.com. I am getting relevant inquiries and also leads after registering my company with them. They are promoting my company and its products all over the country and business is expanding like never before. I will continue my association with them for long!

AnujGupta
METRIX HEALTHCARE INDIA
We have been able to mark a foothold in this domain, just because of GetDistributors. We would always remain thankful to them for helping us grow and how! Ever since we have registered with them, we have received a large number of inquiries, in fact, the phone has never stopped ringing. Every business organization looking for steady growth should register themselves with GetDistributors.

RajeshGanatra
MAY FLOWER INDIA
We appreciate the service rendered by Trade India Portal for our brand and company promotions. We are getting more and more coverage in this global world through this portal. We are proud to be associated since last three years with them and we are totally satisfied with their services. Such as CRM Support which was given by the team regularly. We are thankful for the support on regular basis. Best Luck
Probiotic Sachets Price List
This Data was Last Updated on 2025-06-30
Probiotic Sachets Manufacturers | Suppliers in India
Company Name | Member Since |
|---|---|
3s Corporation Mumbai, India | 14 Years |
Dheer Healthcare Private Limited Mumbai, India | 13 Years |
Lexicare Pharma Pvt. Ltd. Ankleshwar, India | 13 Years |
Nu Alter Remedies Panchkula, India | 13 Years |
Rajvi Enterprise Ahmedabad, India | 13 Years |
Salvavidas Pharmaceutical Pvt. Ltd. Surat, India | 11 Years |
Indo Rama Pharma Nahan, India | 11 Years |
Nutra Healthcare Private Limited Surat, India | 10 Years |
Novalab Health Care Pvt. Ltd. Panchkula, India | 10 Years |
Vea Impex (i) Private Limited Mumbai, India | 9 Years |
Upcoming Tradeshows
HortiProIndia 2025
Thu, 13 Nov, 2025 - Sun, 16 Nov, 2025
drinktec India 2025
Thu, 13 Nov, 2025 - Sat, 15 Nov, 2025
Truck Trailer & Tyre Expo 2025
Tue, 16 Sep, 2025 - Thu, 18 Sep, 2025
The Harit Bharat Expo 2025
Fri, 25 Jul, 2025 - Sun, 27 Jul, 2025
Aesthetic Masterclass 2025
Thu, 28 Aug, 2025 - Fri, 29 Aug, 2025
World of Hospitality Expo 2025
Sun, 10 Aug, 2025 - Tue, 12 Aug, 2025
Warehouse Logistics & Material Handling Expo 2025
Tue, 16 Sep, 2025 - Thu, 18 Sep, 2025
WORLD OF CONCRETE INDIA 2025
Wed, 08 Oct, 2025 - Fri, 10 Oct, 2025
MachAuto 2025
Fri, 25 Jul, 2025 - Mon, 28 Jul, 2025
Renewable Energy India Expo 2025
Thu, 30 Oct, 2025 - Sat, 01 Nov, 2025
Popular Categories