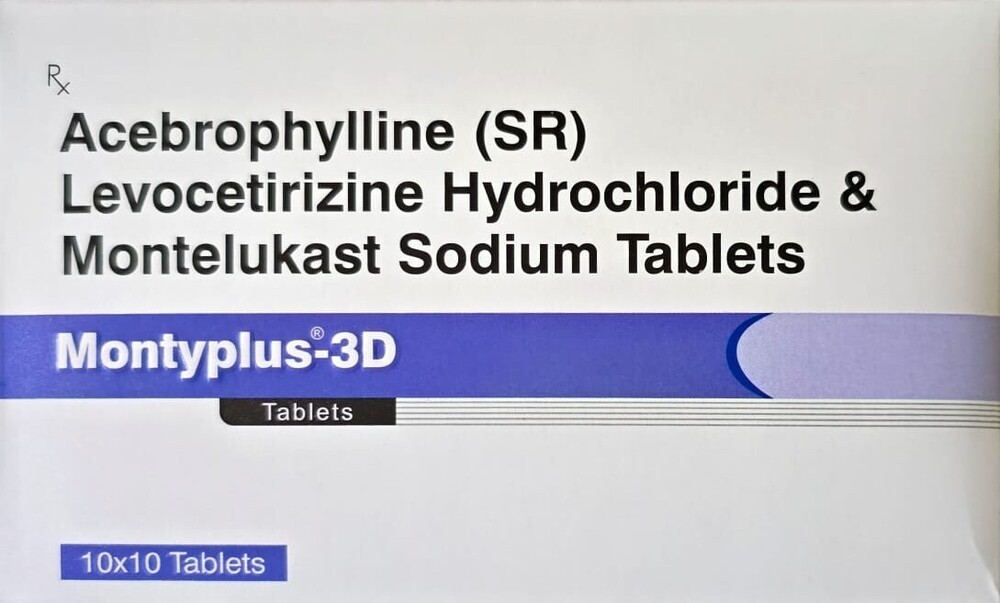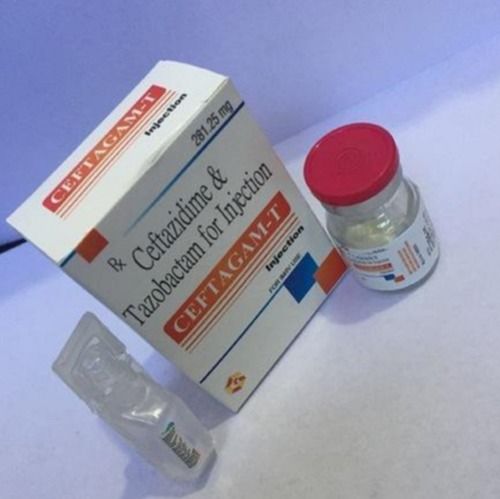
Ceftazidime & Tazobactam Injection
Price:
Get Latest Price
Brand Name :
Ceftabactum
In Stock
Product Specifications
| Salt Composition | Ceftazidime Pentahydrate and Tazobactam Sodium |
| Assay | 98% - 102% (Ceftazidime and Tazobactam combined) |
| Medicine Type | Antibiotic |
| Origin of Medicine | Synthetic |
| Appearance | White to off-white powder |
| Dosage Form | Injection |
| Expiration Date | 24 months from the date of manufacture |
| Grade | Pharmaceutical Grade |
| Storage | Store below 25AdegC in a dry place protect from light |
| Usage | Administered intravenously or intramuscularly under medical supervision |
| Molecular Formula | C22H22N6O7S2 (Ceftazidime) C10H12N4O5S (Tazobactam) |
| CAS No | 78439-06-2 (Ceftazidime) 89785-84-2 (Tazobactam) |
| Indication | Treatment of bacterial infections specifically Gram-negative bacteria including pneumonia urinary tract infections intra-abdominal infections and sepsis |
| Pacakaging (Quantity Per Box) | 10 vials |
| Dosage | Variable depending on indication and severity typically 1-2g Ceftazidime with 0.125-0.25g Tazobactam every 8-12 hours |
Company Details
Business Type
Manufacturer, Supplier
Employee Count
14
Establishment
2011
Working Days
Monday To Sunday
GST NO
08AAGCP0181N1Z4
Payment Mode
Cash in Advance (CID)
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 08AAGCP0181N1Z4
Jaipur, Rajasthan
Director
Mr Abhishek Jain
Members since
9 Years
Address
Plot No-D/194, 2nd Floor, Bapu Nagar, Near Moti Park, Jaipur, Rajasthan, 302015, India
tazobactam injection in Jaipur
Report incorrect details