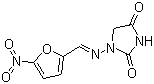
Cefixime Trihydrate - Pharmaceutical Grade Synthetic Antibiotic | Versatile Application, Broad Spectrum Efficacy, Reliable Quality
Price: 100 INR / Kilograms
(100 INR + GST)
Get Latest Price
In Stock
Product Specifications
| Particle Size | Typically <20 microns |
| Color | White |
| Taste | Bitter |
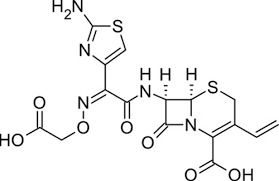
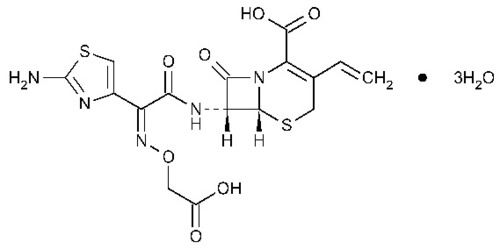
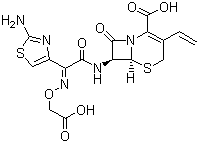
| Molecular Formula | C16H15N5O7S2A*3H2O |
| Shelf Life | 3 years |
| Smell | Other, Odorless |
| Ph Level | 4.5 - 6.5 (in solution) |
| Structural Formula | Refer to image |
| Boiling point | Not applicable (decomposes) |
| Poisonous | Other, Non-poisonous under prescribed dosage |
| HS Code | 29419090 |
| Loss on Drying | a%0$?4.5% |
| Storage | Other, Store in a cool, dry place, tightly closed |
| Heavy Metal (%) | a%0$?0.001% |
| Melting Point | >220AdegC (decomposes) |
| Molecular Weight | 507.50 g/mol |
| Solubility | Sparingly soluble in water; freely soluble in N,N-dimethylformamide; very slightly soluble in ethanol (96%) and in methanol |
| Medicine Name | Cefixime Trihydrate |
| Chemical Name | (6R,7R)-7-{[2-(2-amino-1,3-thiazol-4-yl)-2-(carboxymethoxyimino)acetyl]amino}-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid trihydrate |
| CAS No | 104376-79-6 |
| Type | Other |
| Grade | Other |
| Usage | Used as an oral third-generation cephalosporin antibiotic |
| Purity(%) | >99% |
| Appearance | White to off-white crystalline powder |
| Physical Form | Solid |
| Supply Ability | 2000 Per Day |
| Delivery Time | 1 Days |
| Color | White |
Company Details
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
2
Establishment
2017
Working Days
Monday To Sunday
GST NO
24AKEPV9396A1ZU
Payment Mode
Cash Advance (CA), Telegraphic Transfer (T/T)
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AKEPV9396A1ZU
Ahmedabad, Gujarat
Proprietor
Mr Mayank Shah
Members since
8 Years
Address
16, Vijaynagar Housing Society, Nr. Ved Mandir, Kankaria Ahmedabad, Gujarat, 380022, India
cefixime trihydrate in Ahmedabad
Report incorrect details