Biocompatibility Studies
Price:
Get Latest Price
In Stock
Product Specifications
| Methods | In-vivo, In-vitro |
| Tests | Cytotoxicity, Systemic toxicity |
| Standards | ISO 10993 |
| Reporting | Detailed report |
| Turnaround | 4-6 weeks |
| Usage | Medical device biocompatibility assessment, confirming the absence of harmful effects from medical devices and leachables. |
| Sample type | Various |
| Features | Toxicity testing, Safety assessment, Regulatory compliance, Biocompatible materials, In-vivo/In-vitro, Reliable results, Device safety |
Product Overview
Key Features
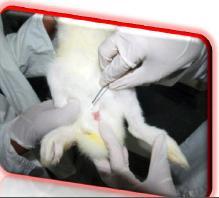
* In-Vivo method: Systemic/Intracutaneous toxicity/Implantation
* In- Vitro method: Cytotoxicity
Company Details
Focusing on a customer-centric approach, INSTITUTE FOR INDUSTRIAL RESEARCH & TOXICOLOGY has a pan-India presence and caters to a huge consumer base throughout the country. Get Others from INSTITUTE FOR INDUSTRIAL RESEARCH & TOXICOLOGY at Trade India quality-assured services.
Business Type
Manufacturer, Service Provider, Supplier
Establishment
2000
Working Days
Monday To Sunday
GST NO
09AABTI4874P1ZV
More Product From This seller
Seller Details

GST - 09AABTI4874P1ZV
Aligarh, Delhi
Director
Mr. N. N. Mishra
Address
BNo F209, PHASE 1, UPSIDC, M .G .ROAD, Aligarh, Uttar Pradesh, 201001, India
Report incorrect details