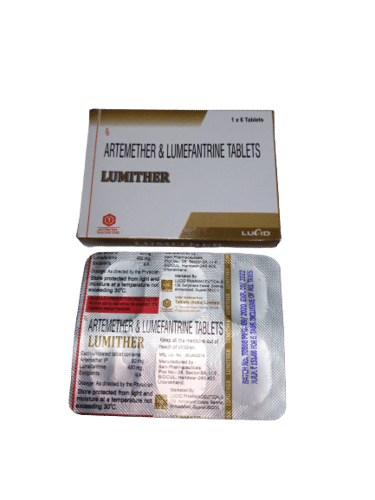
Artemether Lumefantrine Tablet - Antimalarial Treatment, 20 Mg Artemether + 120 Mg Lumefantrine | Effective Against Plasmodium Falciparum Infections
Price: 156.00 INR / Tablet
(156.00 INR + 0% GST)
Get Latest Price
1 Pack Contains :
1
Minimum Pack Size :
10000
In Stock
Product Specifications
| Salt Composition | Artemether 20 mg + Lumefantrine 120 mg (ratios may vary per manufacturer) |
| Origin of Medicine | Synthetic |
| Pacakaging (Quantity Per Box) | 10 blister packs containing 10 tablets each |
| Drug Type | Other, Antimalarial |
| Ingredients | Artemether and Lumefantrine |
| Physical Form | Other, Tablet |
| Function | Other, Treatment of malaria caused by Plasmodium falciparum |
| Recommended For | Individuals diagnosed with malaria infections caused by Plasmodium falciparum |
| Dosage | As prescribed by the healthcare professional (typically twice daily for 3 days) |
| Dosage Guidelines | Take orally with food to enhance absorption. Do not skip doses and complete the full course of treatment even if symptoms improve. |
| Suitable For | Other, Adults and children above a certain weight or age as determined by a physician |
| Quantity | 10 tablets per blister pack |
| Storage Instructions | Store below 30AdegC in a dry place away from direct sunlight and out of reach of children. |
| Currency | INR |
| Price | 156.00 INR (Approx.) |
| Mop | 10000 |
| Price Type | fixed |
| Returnable | No |
| Brand Name | DINATE-CP SYP |
| Product Unit | 10000 Piece/Pieces |
| Stock Quantity | 50000 |
| Moq | 10000 |
| Unit Type | Piece/Pieces |
| Packsize | 1 |
| Minimum Ordered Packs | 10000 |
| Minimum Order Quantity | 10000 |
| GSTIN | 0% |
Company Details
Business Type
Exporter, Manufacturer, Supplier, Wholesaler
Employee Count
100
Establishment
1997
Working Days
Monday To Saturday
GST NO
24AADCD9685J1Z6
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AADCD9685J1Z6
Ahmedabad, Gujarat
Marketing Manager
Mr Kalp Patel
Address
73, Silver Infra Hub, B/h Sakar Health Care, Changodar, Taluka Sanand, Ahmedabad, Gujarat, 382213, India
Report incorrect details