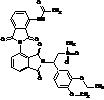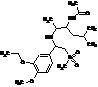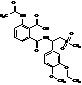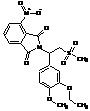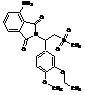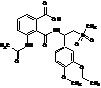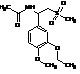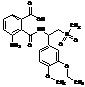
Apremilast Impurity F-2
Price:
Get Latest Price
In Stock
Product Specifications
| Molecular Formula | C20H24N2O7S |
| Molecular Weight | 436.49 |
| Purity | >98% |
| Appearance | White powder |
| Solubility | Water soluble |
| Storage | Room temperature |
| Usage | Apremilast synthesis intermediate. Used in pharmaceutical research and development. |
| Features | High purity, Precise synthesis, Pharmaceutical grade, Reliable source, Cost-effective |
Product Overview
Key Features
MF:C20H24N2O7S
MW: 436.49
Company Details
We are doing custom synthesis of pharmaceuticals Impurities & Reference standards. We also undertake technical consultancy services for R&D and manufacturing departments
Business Type
Exporter, Manufacturer, Service Provider, Supplier, Producer
Employee Count
25
Establishment
2015
Working Days
Monday To Saturday
GST NO
27AAKFC3593J1Z1
Payment Mode
Others
Certification
ISO 9001-2015 / ISO 17024-2016
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 27AAKFC3593J1Z1
Navi Mumbai, Maharashtra
Sales Business Development
Mr Bapu Gawade
Address
Plot No. R-80, 2nd Floor, Prama Instruments, TTC Industrial Area, MIDC, Rabale, Navi Mumbai, Maharashtra, 400701, India
pharmaceutical additives in Navi Mumbai
Report incorrect details



















![Green Quetiapine Impurity H (Quetiapine-N-Oxide)]](https://tiimg.tistatic.com/fp/1/003/453/quetiapine-impurity-h-quetiapine-n-oxide--281.jpg)