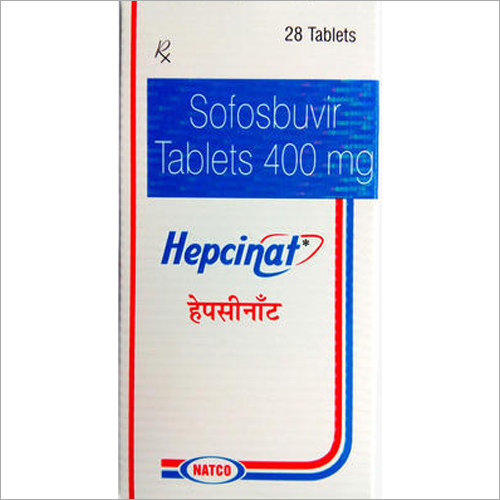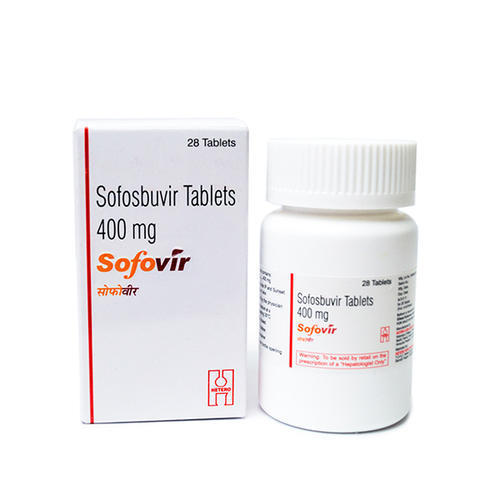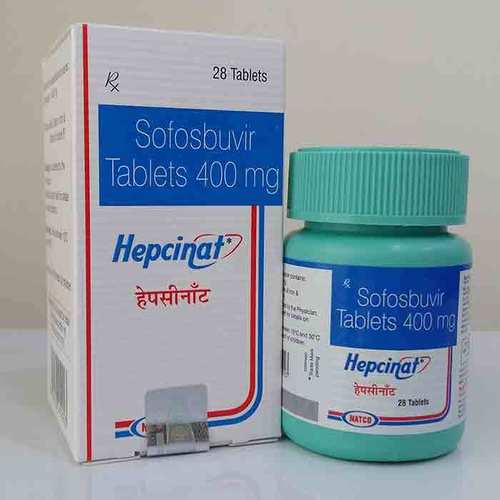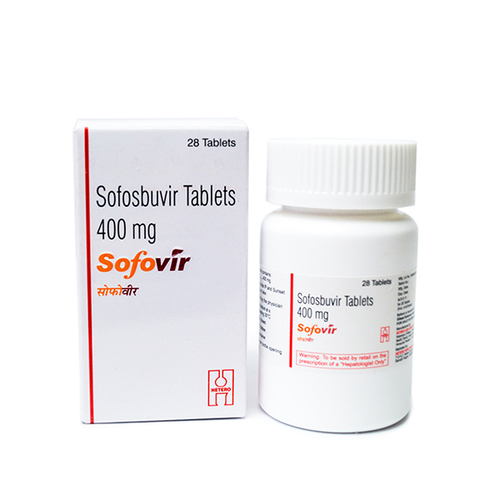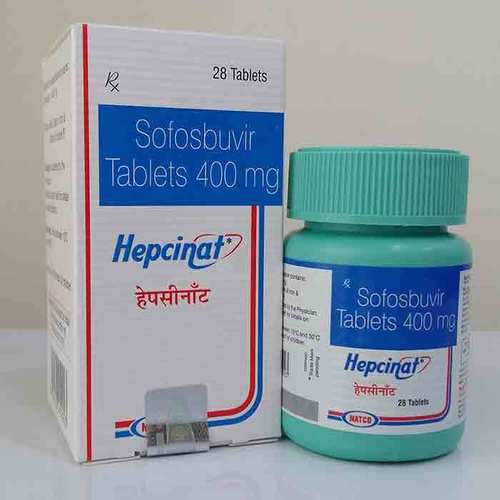

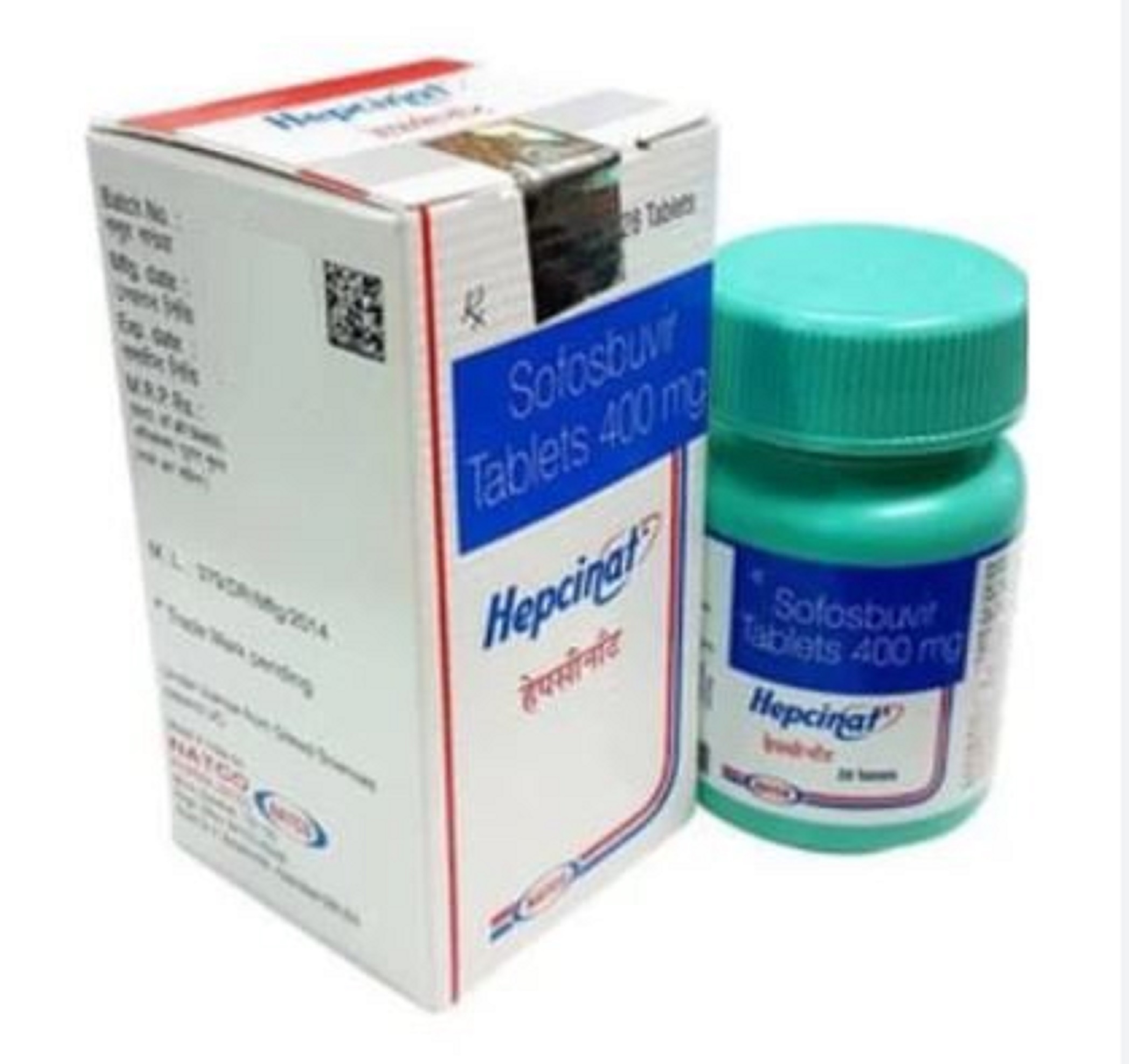

Hepcinat Sofosbuvir 400mg Tablets - Pack Of 28 Tablets | Generic For Hepatitis C Treatment, Manufactured By Natco Pharma, 400mg Strength
Price:
Get Latest Price
Brand Name :
Hepcinat
In Stock
Product Specifications
| Generic Name | Sofosbuvir |
| Brand Name | Hepcinat |
| Dosage | 400mg |
| Packaging | 28 Tablets |
| Form | Tablets |
| Manufacturer | Natco Pharma |
| Usage | Treats Hepatitis C virus infection. Consult physician before use. |
| Features | Hepatitis treatment, Oral medication, 400mg strength, Generic Sofosbuvir, 28 tablets/pack |
| Grade | Pharmaceutical Grade |
| Dosage Form | Oral Tablets |
| Origin of Medicine | India |
| Appearance | Film-coated Tablets |
| Medicine Type | Antiviral Tablets |
| Molecular Formula | C22H29FN3O9P |
| Storage | Store below 30AdegC, protected from moisture |
| Salt Composition | Sofosbuvir 400mg |
| CAS No | 1190307-88-0 |
| Pacakaging (Quantity Per Box) | 28 Tablets |
| Assay | Not less than 98% |
| Indication | Chronic Hepatitis C Virus (HCV) infection |
| Expiration Date | 24 Months from manufacturing date |
| Packaging Details | PACK OF 28 TABLETS |
| Main Domestic Market | All India |
Product Overview
Key Features
Company Details
Focusing on a customer-centric approach, RADHAKISHAN PHARMACEUTICALS has a pan-India presence and caters to a huge consumer base throughout the country. Buy Anti Infective Drugs & Medicines in bulk from RADHAKISHAN PHARMACEUTICALS at Trade India quality-assured products.
Business Type
Exporter, Distributor, Supplier, Trading Company, Wholesaler
Employee Count
40
Establishment
2011
Working Days
Monday To Saturday
GST NO
07AFIPJ3910R1Z2
Certification
ISO 9001:2015 CERTIFIED
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AFIPJ3910R1Z2
Delhi, Delhi

Accepts only Foreign inquiries
Proprietor
Mr. Deepak Jindal
Members since
15 Years
Address
Shop No.1, Second Floor, Property No. 4634/1, Plot No-19, Ward No.11, Ansari Road, Daryaganj, Delhi, Delhi, 110002, India
sofosbuvir tablets in Delhi
Report incorrect details