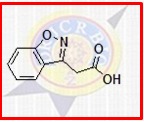
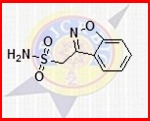
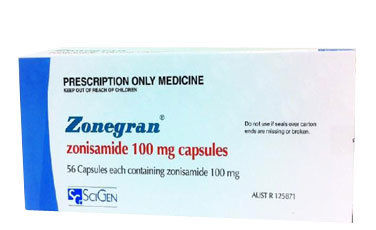
Zonisamide - Adjunctive Treatment For Partial Seizures In Adults , Expertly Formulated By Medical Specialists
Price:
Get Latest Price
In Stock
Product Specifications
| Dosage Form | Capsules |
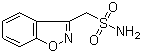
| Active Ingredient | Zonisamide |
| Strength | Variable |
| Packaging | Bottles |
| Storage | Controlled |
| Usage | Adjunctive treatment of partial seizures in adults. |
| Regulatory Compliance | FDA-approved |
| Features | Seizure control, Adjunctive therapy, Oral dosage, Effective treatment, Reliable efficacy |
Product Overview
Key Features
Company Details
Focusing on a customer-centric approach, Apithecary Pharma Pvt. Ltd. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Pharmaceutical Ointments & Creams in bulk from Apithecary Pharma Pvt. Ltd. at Trade India quality-assured products.
Business Type
Exporter, Manufacturer, Supplier
Establishment
2005
Working Days
Monday To Sunday
Related Products
More Product From This seller
Seller Details
Hyderabad, Telangana
Marketing
Mr. Venugopal
Address
Flat No: 3, Samyukta Residency, Near Suchitra Circle, Jeedimetla, Hyderabad, Telangana, 500055, India
zonisamide in Hyderabad
Report incorrect details