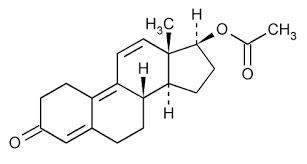
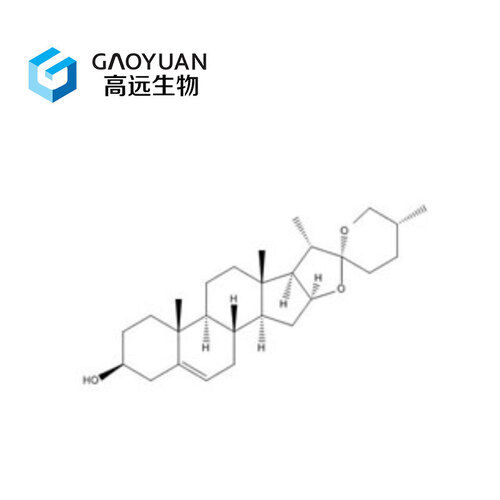
Tetraene Acetate - Molecular Weight 366.45 G/mol White Crystalline Powder | Gmp Standard Production Medicine Grade Cas No 37413-91-5 High Quality Mass Production Expertise
Price:
Get Latest Price
Minimum Order Quantity :
1
In Stock
Product Specifications
| Molecular Formula | C23H26O4 |
| Color | White |
| Storage | Dry Place |
| CAS No | 37413-91-5 |
| Grade | Medicine Grade |
| Physical Form | Powder |
| Main Domestic Market | All India |
Product Overview
Key Features
Molecular Weight:366.45
Appearance:White crystalline powder
GMP standard production lines
Our Advantage:mass production; high quality; competitive price; more than 20 years experiences
Appearance:White crystalline powder
GMP standard production lines
Our Advantage:mass production; high quality; competitive price; more than 20 years experiences
Company Details
XI`AN GAOYUAN BIO-CHEM CO. LTD. Established in 2002 at Xian in Shaanxi is a leading ExporterManufacturerDistributorSupplierTrading Company of Pharmaceutical Raw Materials & Ingredients in China. XI`AN GAOYUAN BIO-CHEM CO. LTD. is one of Trade India's verified and trusted sellers of listed products. With extensive experience in supplying and trading Tetraene Acetate XI`AN GAOYUAN BIO-CHEM CO. LTD. has made a reputed name for itself in the market with high-quality 99% Pure Progesterone USP White Powder Aescin Powder Alendronate Sodium Powder (CAS No.:121268-17-5) etc.
Focusing on a customer-centric approach XI`AN GAOYUAN BIO-CHEM CO. LTD. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Pharmaceutical Raw Materials & Ingredients in bulk from XI`AN GAOYUAN BIO-CHEM CO. LTD. at Trade India quality-assured products.
Focusing on a customer-centric approach XI`AN GAOYUAN BIO-CHEM CO. LTD. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Pharmaceutical Raw Materials & Ingredients in bulk from XI`AN GAOYUAN BIO-CHEM CO. LTD. at Trade India quality-assured products.
Business Type
Exporter, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
100
Establishment
2002
Working Days
Monday To Sunday
Related Products
More Product From This seller
Seller Details
Xian, Shaanxi
Mr. Jason Cao
Address
R11702 Block B Greenland Soho Zhangba Road Xi`An Hi-Tech Zone Xian Shaanxi 710065 China
Report incorrect details