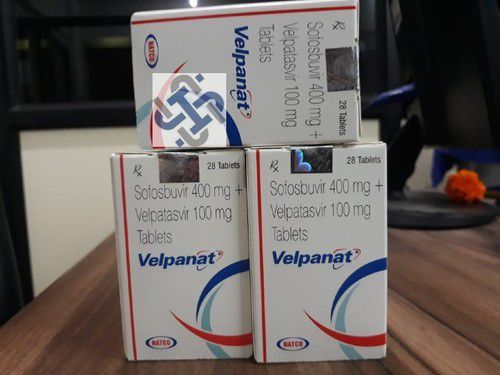
Sofosbuvir And Velapatasvir Tablets - Expiration Date: 3 Years
Price: 5376.00 INR / Bottle
(4800.00 INR + 12% GST)
Get Latest Price
MRP:
5500.00 INR / Bottle
Weight :
0.65 Gram
1 Pack Contains :
28
Minimum Pack Size :
200
In Stock
Product Specifications
| Dosage | As directed by the Physician |
| Salt Composition | Sofosbuvir and Velpatasvir |
| Pacakaging (Quantity Per Box) | 28 tablets in a bottle |
| Medicine Type | Allopathic |
| Origin of Medicine | India |
| Grade | Medical Grade |
| Usage | Treatment of chronic Hepatitis C virus (HCV) infection in adults and children (typically 3 years and older). |
| Dosage Form | Tablet |
| Indication | Treatment of chronic Hepatitis C virus (HCV) infection in adults and children (typically 3 years and older). |
| Expiration Date | 3 Years |
| Storage | Store protected from moisture, at a temperature not exceeding 30 c |
| FOB Port | Ahmedabad |
| Payment Terms | Paypal, Cash Advance (CA), Cash in Advance (CID), Cheque, Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Others, Letter of Credit (L/C) |
| Supply Ability | 10000 Per Month |
| Delivery Time | 1 Week |
| Sample Available | Yes |
| Sample Policy | Sample costs shipping and taxes has to be paid by the buyer |
| Packaging Details | 28 tablets in a bottle |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East |
| Main Domestic Market | All India |
| Certifications | WHO GMP |
| Pkg Box Length | 4.00 cm |
| Stock Quantity | 10000 |
| Brand Name | Velakast |
| Pkg Box Breadth | 4.00 cm |
| GSTIN | 12% |
| Weight | 0.65 Gram |
| Price | 4800.00 INR (Approx.) |
| Packsize | 28 |
| Unit Type | Bottle/Bottles |
| Returnable | No |
| Moq | 200 |
| Shipping Type | free |
| MRP | 5500.00 INR |
| Mop | 200 |
| Product Unit | 200 Bottle/Bottles |
| Price Type | fixed |
| Currency | INR |
| Pkg Box Height | 6.00 cm |
| Minimum Order Quantity | 200 |
| Minimum Ordered Packs | 200 |
Product Overview
Key Features
How It Works
Velakast contains two different direct-acting antiviral (DAA) agents that work together to combat the Hepatitis C virus:
Sofosbuvir (NS5B Polymerase Inhibitor): This drug is a "pro-drug" that gets converted into its active form inside the body. It then interferes with the NS5B protein, which is an enzyme essential for the virus to copy its RNA and replicate itself. By inhibiting this process, sofosbuvir effectively stops the virus from multiplying.
Velpatasvir (NS5A Inhibitor): This drug targets a different viral protein called NS5A. The NS5A protein is crucial for both viral replication and the assembly of new virus particles. By blocking its function, velpatasvir further disrupts the virus's life cycle.
The combination of these two mechanisms of action makes Velakast highly potent and effective against all six major genotypes of the Hepatitis C virus. This dual-target approach helps to reduce the risk of the virus developing resistance to the medication.
Uses and Indications
Velakast is primarily used to treat chronic Hepatitis C in adults and children aged 3 years and older. Its effectiveness extends to patients with or without cirrhosis (liver scarring), including those with compensated cirrhosis (Child-Pugh A).
For patients with decompensated cirrhosis (Child-Pugh B or C): Velakast is often prescribed in combination with another antiviral medication called ribavirin.
The typical duration of treatment is 12 weeks, though this can vary depending on the patient's specific condition, HCV genotype, previous treatment history, and the presence of cirrhosis.
Dosage and Administration
Standard Dose: The usual dose for adults is one tablet (400 mg sofosbuvir and 100 mg velpatasvir) taken once daily.
Administration: The tablet should be swallowed whole, with or without food. It should not be crushed, broken, or chewed.
Missed Dose: If a dose is missed, it should be taken as soon as it is remembered. However, if it is almost time for the next dose, the missed dose should be skipped to avoid taking a double dose. It's important to maintain a consistent daily schedule.
Important Warnings and Precautions
Before and during treatment with Velakast, a doctor must be made aware of several key health factors:
Hepatitis B Virus (HBV) Reactivation: All patients should be tested for HBV before starting treatment. In some cases, treating Hepatitis C with DAAs like Velakast can cause the Hepatitis B virus to reactivate, leading to severe liver problems, including liver failure and death. Patients with a history of HBV must be closely monitored.
Serious Bradycardia (Slow Heart Rate): A potentially life-threatening side effect, severe symptomatic bradycardia has been reported when sofosbuvir-containing drugs are used with amiodarone, a medication for irregular heartbeats. Co-administration of these drugs is generally avoided. If necessary, the patient should be closely monitored in a hospital setting for at least the first 48 hours.
Kidney and Liver Disease: While Velakast can be used in patients with mild to moderate kidney impairment, it should be used with caution in those with severe kidney disease or end-stage renal disease. Dosage adjustments may be necessary. It is generally safe for use in patients with liver disease.
Pregnancy: The safety of Velakast during pregnancy is not well-established. If a patient is taking Velakast in combination with ribavirin, effective birth control is critical, as ribavirin can cause severe birth defects.
Common Side Effects
The most frequently reported side effects of Velakast include:
Headache
Fatigue
Nausea
Diarrhea
Insomnia
Irritability
Company Details
We basically dealing in pain killer medicine, anti anxiety and anti depressants, mood alert medicine, anti viral medicine, anti cancer medication, ed medication, Anti HIV medicine and nephrology.
Business Type
Exporter, Service Provider, Supplier, Trading Company
Employee Count
10
Establishment
2015
Working Days
Monday To Sunday
GST NO
24CJWPK9058H1ZI
Payment Mode
Western Union
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24CJWPK9058H1ZI
Ahmedabad, Gujarat

Accepts only Foreign inquiries
Director
Mr Mr Tarun
Members since
2 Years
Address
Shop No. - 12, Ground Floor, TP76/B, Suvarna Residency, B/H Sanidhya Flora, Chandkheda, Ahmedabad, Gujarat, 382424, India
sofosbuvir tablets in Ahmedabad
Report incorrect details