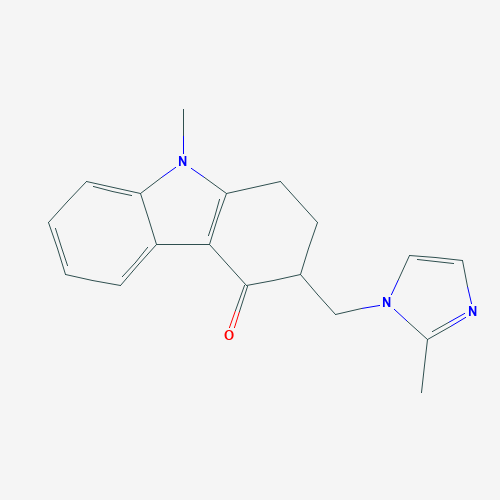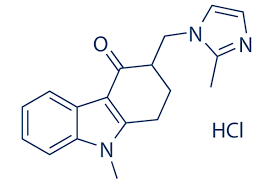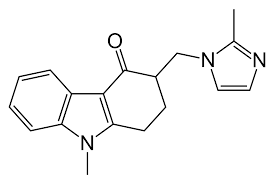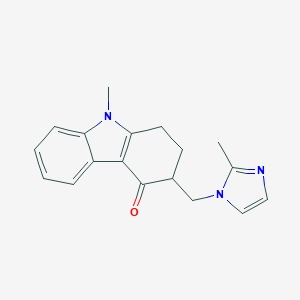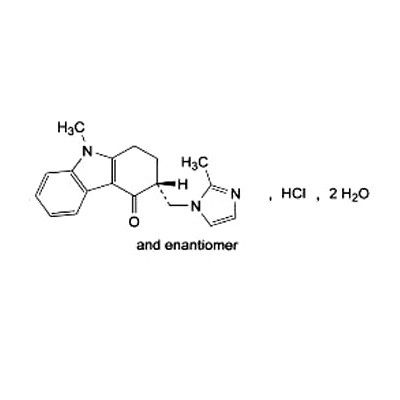
Ondansetron Hcl Bp - C18h20cln3o, 2h2o | High Purity Pharmaceutical Intermediate, Manufactured To International Standards
Price:
Get Latest Price
In Stock
Product Overview
Key Features
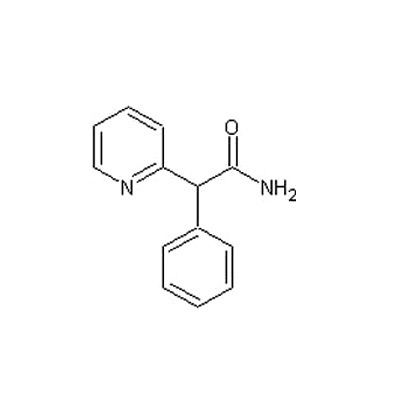
With the use of best quality raw materials we manufacture a great variety of Ondansetron Hcl BP intermediates in international standards. Featured with purity and accuracy in composition these intermediates are manufactured and exported to various pharmaceutical sectors.
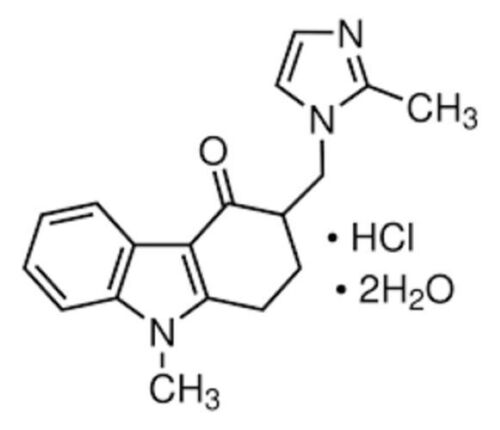
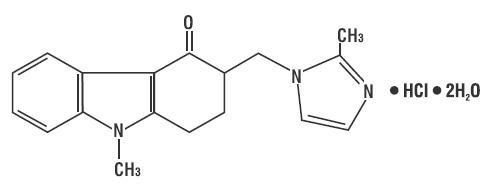
* C.A.S. REG. NO. : 103639-04-9
* MOL. FORMULA : C18H20ClN3O,2H2O
* MOL. WT. : 365.9
* C.A.S. REG. NO. : 103639-04-9
* MOL. FORMULA : C18H20ClN3O,2H2O
* MOL. WT. : 365.9
Company Details
MAHRSHEE LABORATORIES PVT. LTD., Established in 1999 at Bharuch in Gujarat, is a leading Exporter,Manufacturer of Others in India. MAHRSHEE LABORATORIES PVT. LTD. is one of Trade India's verified and trusted sellers of listed products. With extensive experience in supplying and trading Ondansetron HCL, MAHRSHEE LABORATORIES PVT. LTD. has made a reputed name for itself in the market with high-quality Cetirizine Dihydrochloride, Dexchlorpheniramine Maleate, Diphenyl Acetonitrile, etc.
Focusing on a customer-centric approach, MAHRSHEE LABORATORIES PVT. LTD. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Others in bulk from MAHRSHEE LABORATORIES PVT. LTD. at Trade India quality-assured products.
Focusing on a customer-centric approach, MAHRSHEE LABORATORIES PVT. LTD. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Others in bulk from MAHRSHEE LABORATORIES PVT. LTD. at Trade India quality-assured products.
Business Type
Exporter, Manufacturer
Employee Count
20
Establishment
1999
Working Days
Monday To Sunday
GST NO
24AABCM9228N1Z4
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AABCM9228N1Z4
Bharuch, Gujarat
Marketing Manager
Mr. Pragnesh Dobaria
Address
Plot No-3014, 3015, G.i.d.c. Phase-III, Panoli, Bharuch, Gujarat, 394116, India
ondansetron hcl in Bharuch
Report incorrect details