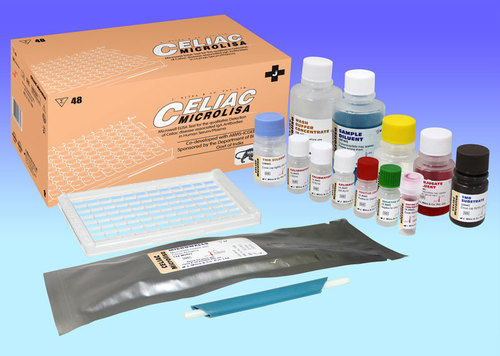
Microlisa Hiv
Price:
Get Latest Price
In Stock
Product Specifications
| Assay type | ELISA |
| Sample type | Serum/plasma |
| Well format | 96-well plate |
| Incubation time | 90 minutes |
| Shelf life | 15 months |
| Storage temp | 2-8°C |
| Antigens | gp41, gp120, gp36 |
| Features | HIV-1 & HIV-2 detection, 90-minute assay, High sensitivity, High specificity, Long shelf life, Color-coded reagents, Group O & subtype C detection |
Product Overview
Key Features
Company Details
Business Type
Exporter, Manufacturer, Supplier
Employee Count
250
Establishment
1969
Working Days
Monday To Saturday
GST NO
07AAACJ0482C2Z0
Related Products
More Product From This seller
Seller Details

GST - 07AAACJ0482C2Z0
New Delhi, Delhi
Director
Mr Jatin Mahajan
Address
A-180-181, Okhla Industrial Area, Phase-I, New Delhi, Delhi, 110020, India
Medical Equipment in New Delhi
Report incorrect details