




Lumefantrine Medicine Raw Materials
Price: 1200 INR / Kilograms
Get Latest Price
Minimum Order Quantity :
5 Kilograms
Brand Name :
Lumefantrine
In Stock
Product Specifications
| Solubility | water is less than 50 ng/mL |
| Storage | Keep away from moisture |
| EINECS No | 617-303-4 |
| Color | yellow |
| Taste | Other |
| Melting Point | 128AdegC to 138AdegC |
| Particle Size | 100 nm or less |
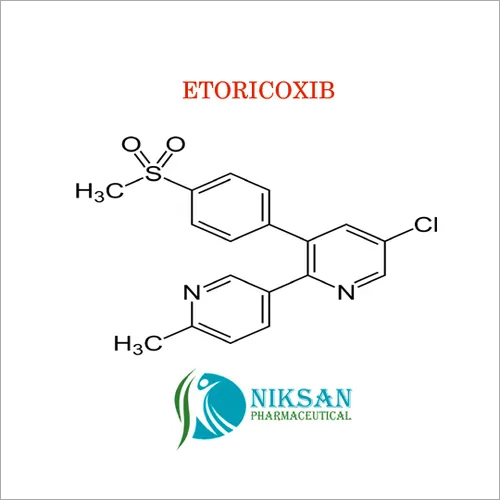
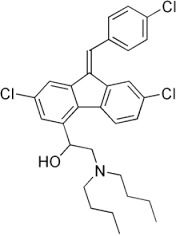
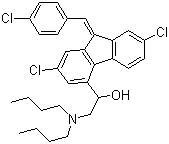
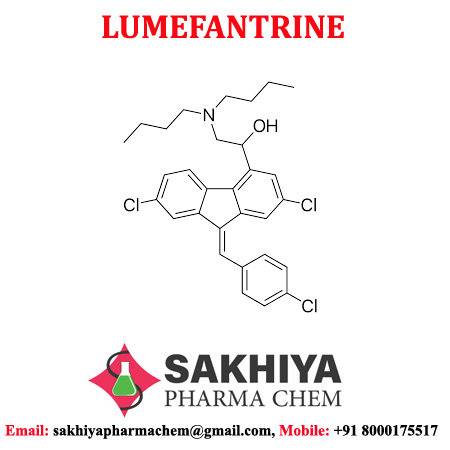
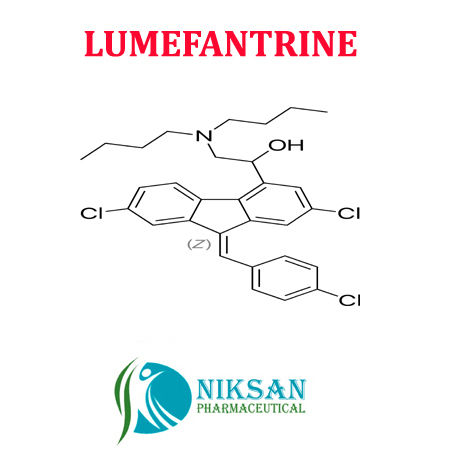
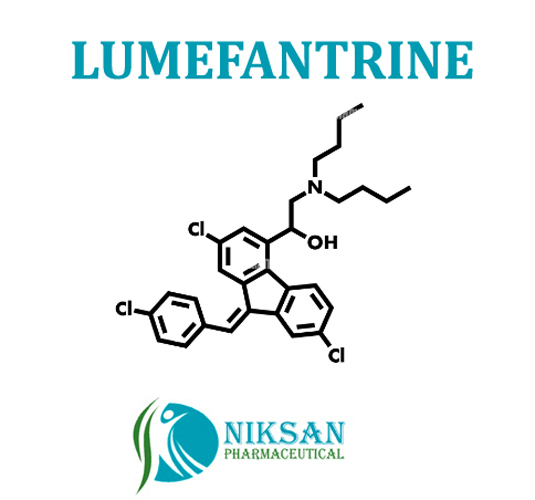
| Structural Formula | C30H32Cl3NO |
| Boiling point | 760 mmHg |
| Loss on Drying | less than 2% |
| HS Code | 29420090 |
| Molecular Weight | 528.94 g/mol Grams (g) |
| Ph Level | 1.2 |
| Molecular Formula | C30H32Cl3NO |
| Shelf Life | 3 Years |
| Smell | Other |
| Medicine Name | LUMEFANTRINE |
| Drug Type | Medicine Raw Materials |
| Ingredients | LUMEFANTRINE |
| Chemical Name | (9Z)-2,7-Dichloro-9-[(4-chlorophenyl)methylene]-I+--[(dibutylamino)methyl]-9H-fluorene-4-methanol |
| CAS No | 82186-77-4 |
| Function | Anti-Malaria |
| Type | Pharmaceutical Intermediates |
| Grade | Other |
| Recommended For | Lumefantrine is the antimalarial drug. Lumefantrine used in the treatment of malaria caused by the mosquito bite. |
| Dosage | tablets, oral solution |
| Usage | Take medication 2 times per day with meal for three day. Take your doctoraEURtms advice before taking the medication. If you vomit after taking the drug, take medicine again after 30 minutes. |
| Purity(%) | 99 % |
| Dosage Guidelines | Take medication 2 times per day with meal for three day. Take your doctoraEURtms advice before taking the medication. If you vomit after taking the drug, take medicine again after 30 minutes. |
| Appearance | White to Off-White Solid |
| Quantity | - Unit |
| Physical Form | Powder |
| Storage Instructions | Store in room temperature in dry place. Do not keep medication in humid place or bathroom. |
| FOB Port | NHAVA SHEVA |
| Payment Terms | Cash Advance (CA), Cheque |
| Supply Ability | 100 Per Week |
| Delivery Time | 1 Days |
| Sample Available | Yes |
| Sample Policy | Free samples are available |
| Packaging Details | HDPE DRUM WITH TWO LDPE INNER LINER |
| Main Export Market(s) | Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa |
| Main Domestic Market | All India |
| Certifications | WHO GMP,GMP,GLP,ISO |
Product Overview
Key Features
Company Details
NIKSAN PHARMACEUTICAL was established by a group of experienced professionals with an objective to develop Pharmaceutical Products in a complex quality driven atmosphere. we put in our the best efforts to provide Active Pharmaceutical Ingredients and Pharmaceutical Finished Formulation in various dosage forms (Tablets, Capsules, Syrups, Gels, Ointments, Nasal Sprays and Dry powder in pouch Pack) with prime focus on the exports of the APIs and Pharmaceutical Finished Formulation. We maintain high level of integrity and transparency in our business processes and dealings and thus assure high quality products to the customers. We are developing high quality Pharmaceutical Intermediate through Research & Development using new technologies. Our R & D team is expert Process Development, process optimization and scale-up to big quantity.
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
26
Establishment
2013
Working Days
Sunday To Monday
GST NO
24AAKFN4352Q1ZR
Payment Mode
Cash Against Delivery (CAD), Cash on Delivery (COD), Cash Advance (CA), Cash in Advance (CID), Cheque, Days after Acceptance (DA), Delivery Point (DP), Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Paypal, Others
Certification
FIRM REGISTRATION
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AAKFN4352Q1ZR
Ankleshwar, Gujarat
Sales Department
Mr Sanjay Patel
Members since
12 Years
Address
Plot No. 4706/03, Gidc Estate, SF-12, Shrinathji Arcade, Near Meghmani Chowkadi, Ankleshwar, Gujarat, 393002, India
active pharmaceutical ingredients in Ankleshwar
Report incorrect details