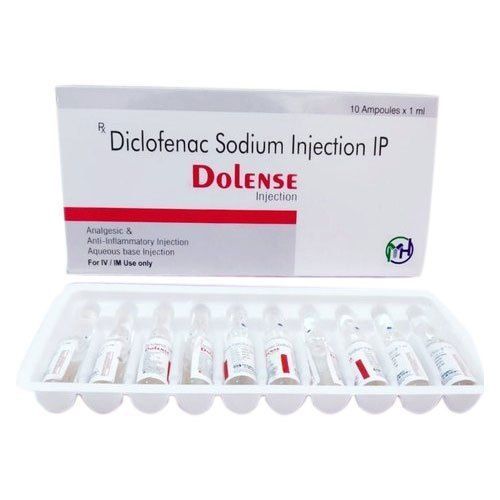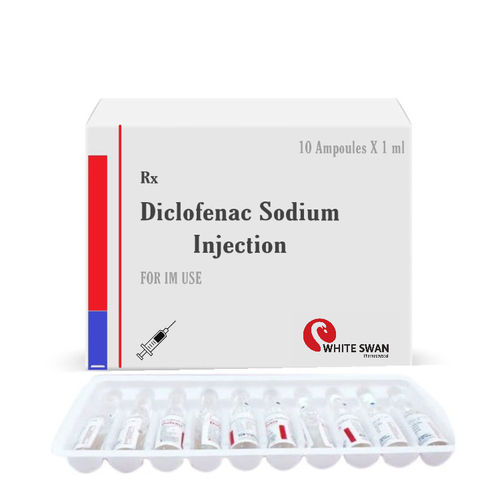
Diclofenac Sodium Injection - 75mg Liquid Form , Sterilized And Multi-use For Pain Relief
Price: 160 INR / Piece
Get Latest Price
Minimum Order Quantity :
100 Piece
Brand Name :
Dixlo-75
In Stock
Product Specifications
| Drug Type | General Medicines |
| Ingredients | DICLOFENAC SODIUM 75MG |
| Physical Form | Liquid |
| Use Type | Multiple Use |
| Recommended For | Suitable For All |
| Quantity | 50 Pieces |
| Storage Instructions | Store in a cool dry place. |
| Sterilized | Yes |
| Disposable | No |
| Recyclable | No |
| FOB Port | New Delhi |
| Payment Terms | Telegraphic Transfer (T/T), Cash in Advance (CID), Cheque, Cash Advance (CA) |
| Delivery Time | 12-14 Days |
| Sample Available | Yes |
| Sample Policy | Contact us for information regarding our sample policy |
| Packaging Details | 10X1ML |
| Main Export Market(s) | Asia |
| Main Domestic Market | Chhattisgarh, Madhya Pradesh, Bihar, Assam, Andhra Pradesh, Tamil Nadu, West Bengal, Telangana, Karnataka, Central India, Kerala, Haryana, Maharashtra, Delhi, North India, Odisha, Jharkhand, Uttar Pradesh, All India |
| Certifications | ISO-GMP CERTIFICATION |
Product Overview
Key Features
Company Details
We would like to introduce ourselves as Manufacturer, Trader and Exporter of all Pharmaceutical products and formulations. We have around 7 years of experience in Manufacturing Tablet, Softgel Capsules, Capsule, Dry Syrup & Oral Liquid under Beta & Non Beta Lactum all under one roof. Our state of the art manufacturing unit is located in Paonta Sahib, Himachal Pradesh and we follow high standard of "Good Manufacturing Practice". The company have GMP and ISO certification.
Business Type
Exporter, Manufacturer, Distributor, Supplier, Trading Company, Wholesaler, Dealer
Employee Count
20
Establishment
2009
Working Days
Monday To Saturday
GST NO
07AGAPG4585E1ZL
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 07AGAPG4585E1ZL
New Delhi, Delhi

Accepts only Domestic inquiries
Gm Sales
Ms Priyanka
Members since
10 Years
Address
C-5/9, Vashist Park, New Delhi, Delhi, 110046, India
Report incorrect details