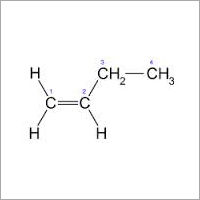
Cis-2-butene
Price:
Get Latest Price
In Stock
Product Specifications
| Purity | 99% |
| MolecularWeight | 56.11 |
| BoilingPoint | 3.72°C |
| Density | 2.46 kg/m³ |
| VaporPressure | 0.88 bar @0°C |
| FlammabilityRange | 1.7-9.7% |
| SpecificVolume | 0.407 m³/kg |
| Features | High purity, Versatile chemical, Wide applications, Reliable source, Cost-effective |
Product Overview
Key Features
| Product information | |
| Quality code | 2.0 . |
| Purity % volume | >=99 |
| Impurities ppm | |
| (where nothing else stated) | |
| trans-2-Butene | 0.5% |
| O2 | 10 |
| N2 | 50 |
| General information Filling pressure at 15 C, bar: | 1.5 |
| Material recommendations: | Gas: No restrictions |
| Liquid: Avoid plastic and rubber. | |
| Characteristics | |
| Highly flammable, liquefied, colourless gas. | |
| Health risks | |
| Asphyxiating. | |
| Transport | |
| ADR Class 2, 3(b). | |
| Physical data | |
| Molecular weight: | 56.11 56.11 |
| Boiling point at 1.013 bar, C: | 3.72 |
| Density (1.013 bar, 15 C) kg/m3: | 2.46 |
| Vapour pressure at 0 C, bar: | 0.88 |
| 20 C, bar: | 1.8 |
| Flammability range in air, % (volume): | 1.7-9.7 |
| Specific volume (1.013 bar, 15 C), m3/kg: | 0.407 |
| Source | |
Almost all commercially produced butenes are obtained as by-products
| |
| The butenes obtained are withdrawn as a mixture from the C4-fraction. From this mixture butadiene and butanes are separated by extractive distillation. The remaining butenes cannot be separated by mere distillation because their boiling points are too close together. | |
| In a first step iso-butene is isolated either by etherification with methanol to form methyl tert-butylether (MTB), or by hydrating iso-butene to tert-butanol (TBA). In this step all other C, components in the mixture remain unchanged. MTB and TEA can then be split by reversing synthesis to produce high purity iso-butene. Once the iso-butene content has been reduced, recovery of high purity 1-butene is possible by fractionation. The remaining 2-butenes can be separated by molecular sieve absorption methods. | |
Other commercial processes that are sometimes used to produce specific isomers or mixtures of butenes or both, either directly or as by-products, include:
AU or any of them may become useful feedstock sources should the need arise. | |
Applications
| |
Company Details
Focusing on a customer-centric approach, SPECIAL GAS & EQUIPMENT SOLUTIONS PVT. LTD. has a pan-India presence and caters to a huge consumer base throughout the country. Buy Industrial Gases in bulk from SPECIAL GAS & EQUIPMENT SOLUTIONS PVT. LTD. at Trade India quality-assured products.
Business Type
Manufacturer, Supplier
Employee Count
5
Establishment
2010
GST NO
19AAOCS4059G1ZW
Related Products
More Product From This seller
Seller Details

GST - 19AAOCS4059G1ZW
Kolkata, West Bengal
Director
Mr. Saibal Basu
Address
Plot No. BJ- 133, Sector II, Salt Lake, near ICICI bank, Kolkata, West Bengal, 700091, India
Industrial Gases in Kolkata
Report incorrect details