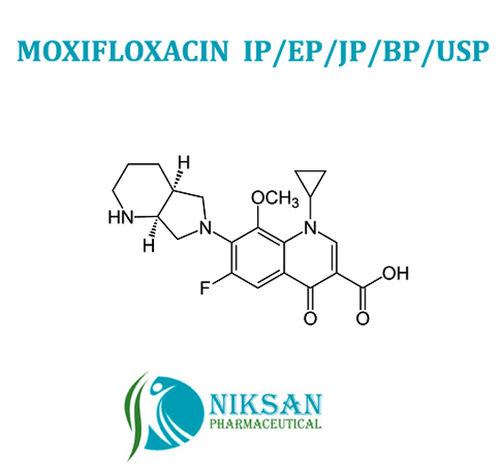
Moxifloxacin Hcl - Cas No. 12-8039, Molecular Formula C21h25clfn3o4 | Broad Spectrum Antibiotic For Pneumonia, Tuberculosis And Conjunctivitis Treatment In Tablets, Creams, Ointments, And Capsules
Price: 14500 INR / Kilograms
Get Latest Price
Minimum Order Quantity :
5KG Kilograms
In Stock
Product Specifications
| Physical Form | Powder |
| Molecular Weight | 437.89 gaEURC/mola^'1 |
| Product Type | API |
| Usage | Moxifloxacin HCL is one type of antibiotic. Moxifloxacin HCL belongs to the class called fluoroquinolone. Moxifloxacin HCL gives antibiotic activity by killing the bacteria. Moxifloxacin HCL used in the treatment of pneumonia, tuberculosis, conjunctivitis. Moxifloxacin HCL available in tablet, creams, ointments and capsules. |
| Molecular Formula | C21H25ClFN3O4 |
| Storage | Room Temperature |
| CAS No | 12-8039 |
| Poisonous | NO |
| Application | medicine |
| Ingredients | Moxifloxacin HCL |
| Grade | MEDICINE |
| FOB Port | sahar air cargo |
| Payment Terms | Cash Against Delivery (CAD), Cash on Delivery (COD), Cash Advance (CA), Days after Acceptance (DA), Delivery Point (DP), Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Western Union, Paypal, Others, Telegraphic Transfer (T/T), Cash in Advance (CID), Cheque |
| Supply Ability | 100 Per Week |
| Delivery Time | 15 Days |
| Sample Available | Yes |
| Sample Policy | Sample costs shipping and taxes has to be paid by the buyer |
| Packaging Details | DOUBLE LDPE LINERS WITH HDPE CARBOYS |
| Main Export Market(s) | Australia, Eastern Europe, Western Europe, Middle East, Central America, South America, Asia, North America, Africa |
| Main Domestic Market | Dadra and Nagar Haveli, Himachal Pradesh, Andaman and Nicobar Islands, Uttarakhand, Daman and Diu, Lakshadweep, South India, North India, East India, Andhra Pradesh, Assam, Arunachal Pradesh, Bihar, Chandigarh, Goa, Jammu and Kashmir, Karnataka, Madhya Pradesh, Mizoram, Meghalaya, Manipur, Pondicherry, Rajasthan, Sikkim, Tamil Nadu, Tripura, West Bengal, Nagaland, Maharashtra, Haryana, Gujarat, Delhi, Punjab, Telangana, Kerala, Central India, Odisha, Jharkhand, West India, Chhattisgarh, Uttar Pradesh, All India |
| Certifications | GMP, GLP, FDA, ISO |
Product Overview
Key Features
Niksan Pharmaceutical exporting very big quantity of the fine quality products of Moxifloxacin HCL in all over world for many years in a countries like Canada, USA, Romania, Finland, Malaysia, Netherland, South Africa, Brazil, Egypt, Singapore, Jordan, Lebanon, Israel, Vietnam, Bangladesh, Paraguay, Argentina, Dominican Republic, Sudan, Hong Kong, Seychelles, Algeria, Iran, Uruguay, Russia, Thailand, Afghanistan, Latvia, Lithuania, United Arab Emirates, Seychelles, Peru, Switzerland, Tunisia, France, Hungary, Finland, Turkey, Pakistan, Rwanda, South Africa, Denmark, Malawi, Croatia, Slovenia, Ireland, Zambia, Cyprus, Nigeria, Uzbekistan, Cameroon, Netherlands, Azerbaijan, Venezuela, Morocco, Cote D Ivoire, St Lucia, South Korea, Congo, Philippines, Colombia Sweden, Hungary, Mauritius, Vanuatu, Malta, Kazakistan, Slovenia, Bolivia, Japan, Uganda, Australia and many more countries.
Niksan Pharmaceutical also supplies large quantity of Moxifloxacin HCL products in Indian states like Gujarat, Haryana, Rajasthan, Madhya Pradesh, Kerala, Tamilnadu, Delhi, Bihar, Uttar Pradesh, Assam, Goa, Hyderabad, Telangana, Mizoram, Sikkim etc.
Moxifloxacin HCL is one type of antibiotic. Moxifloxacin HCL belongs to the class called fluoroquinolone. Moxifloxacin HCL gives antibiotic activity by killing the bacteria. Moxifloxacin HCL used in the treatment of pneumonia, tuberculosis, conjunctivitis.
Moxifloxacin HCL available in tablet, creams, ointments and capsules.
SYNONYMS: Moxifloxacin HCL, Moxifloxacino HCL
IUPAC NAME: 1-Cyclopropyl-6-fluoro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid hydrochloride
CAS NO: 12-8039
MOLECULAR FORMULA: C21H25ClFN3O4
MOLECULAT MASS: 437.89 gAcA AcmolAcA A 1
STORAGE CONDITIONS: Store in cool and dry place, away from direct sun light and heat. Do not store medicine in bathroom or any humid place. Keep away from children and pets.
HOW TO USE: Take medicine orally with or without food once per day. Wash eyes and remove the lance before using Moxifloxacin ophthalmic solution. Do not consume the ophthalmic solution. Take your doctorAcA A s advice if you have any confusion related to the medicine.
HOW MOXIFLOXACIN HCL WORKS: Moxifloxacin inhibits the topoisomerase II and topoisomerase IV enzyme. By inhibiting these enzymes Moxifloxacin inhibits the replication of DNA. And also inhibits the cell division of the bacteria.
PHARMACOKINETICS: Moxifloxacin absorbed rapidly after oral administration having bioavaibility of 90%. Almost 50% of drug binds with plasma proteins. Half life of Moxifloxacin is between 11.5-15.6 hours. Approximately 45% of Moxifloxacin drug eliminated through urination and feces.
SIDE EFFECTS OF MOXIFLOXACIN: The commons side effects of Moxifloxacin are nausea, vomiting, headache, insomnia, weakness, dizziness are seen in patients. If you see signs of kidney problem, liver problem, GI problem like bleeding, stomach pain, abdominal discomfort, dark urine seek immediate medical attention. Moxifloxacin also cause some intestinal problem like bloody stool, abdominal pain, diarrhoea, cramping in intestine.
PRECAUTION: Tell your doctor if you have allergic reaction to Moxifloxacin medicine. If you have problem like liver problem, heart problem, depression, brain problem like AlzheimerAcA A s, blood vessels problem, mood disorders, brain tumours, or any blood pressure problem tell your doctor before taking the medication. Moxifloxacin can increase the heart problems so kindly avoid the medicine if you have any heart related problems.
CDSCO APPROVAL: Moxifloxacin (0.5%) + Dexamethasone Phosphate (0.1%) Eye drops approved by CDSCO in India in 24.11.2005,
Moxifloxacin HCL (0.5%) + Ketorolac Tromethamine 0.5% Ophthalmic Solution approved by CDSCO in India in 11.07.2007,
Moxifloxacin tab. approved by CDSCO in India in 27.12.2001,
Difluprednate 0.05% w/v + Moxifloxacin 0.5% w/v Eye Drops approved by CDSCO in India in 22.09.2011
Moxifloxacin intravenous infusion approved by CDSCO in India in 13.02.2002
Moxifloxacin HCl (5mg) + Prednisolone Acetate (10mg) per ml. (eye drops) approved by CDSCO in India in 16.10.2007
Ketorolac Tromethamine 0.4% w/v + Moxifloxacin 0.5% w/v ophthalmic solution approved by CDSCO in India in 21.09.2010
Loteprednol etabonate 0.5% w/v + Moxifloxacin 0.5% w/v ophthalmic Suspension approved by CDSCO in India in 01.12.2009
Moxifloxacin ophthalmic solution approved by CDSCO in India in 19.10.2007
Moxifloxacin eye ointment approved by CDSCO in India in 01.10.2008
Moxifloxacin HCl approved by CDSCO in India in 06.06.2001
Moxifloxacin HCl BP 0.5% w/v + Bromfenac Sodium 0.09% w/v Eye drop approved by CDSCO in India in 05.01.2011
Moxifloxacin ophthalmic solution 0.5% approved by CDSCO in India in 27.08.2004
Moxifloxacin Eye Ointment (5mg/gm) approved by CDSCO in India in 06.02.2006
Cefixime (SR) 400mg + Moxifloxacin (SR) 400 mg Tablets approved by CDSCO in India in 08.08.2011
FORMULATIONS AVAILABLE IN MARKET:
Moxifloxacin (0.5%) + Dexamethasone Phosphate (0.1%) Eye drops
Moxifloxacin HCL (0.5%) + Ketorolac Tromethamine 0.5% Ophthalmic Solution
Moxifloxacin tablets
Difluprednate 0.05% w/v + Moxifloxacin 0.5% w/v Eye Drops
Moxifloxacin intravenous infusion
Moxifloxacin HCl (5mg) + Prednisolone Acetate (10mg) per ml. (eye drops)
Ketorolac Tromethamine 0.4% w/v + Moxifloxacin 0.5% w/v ophthalmic solution
Loteprednol etabonate 0.5% w/v + Moxifloxacin 0.5% w/v ophthalmic Suspension
Moxifloxacin HCl BP 0.5% w/v + Bromfenac Sodium 0.09% w/v Eye drop
Moxifloxacin Eye Ointment (5mg/gm)
Cefixime (SR) 400mg + Moxifloxacin (SR) 400 mg Tablets
Note: Product protected by valid patents are not offered for sale in countries where such patents are still valid and its liability is at Buyers Risk.
REFERENCES:
https://pubchem.ncbi.nlm.nih.gov
https://www.wikipedia.org/
Company Details
NIKSAN PHARMACEUTICAL was established by a group of experienced professionals with an objective to develop Pharmaceutical Products in a complex quality driven atmosphere. we put in our the best efforts to provide Active Pharmaceutical Ingredients and Pharmaceutical Finished Formulation in various dosage forms (Tablets, Capsules, Syrups, Gels, Ointments, Nasal Sprays and Dry powder in pouch Pack) with prime focus on the exports of the APIs and Pharmaceutical Finished Formulation. We maintain high level of integrity and transparency in our business processes and dealings and thus assure high quality products to the customers. We are developing high quality Pharmaceutical Intermediate through Research & Development using new technologies. Our R & D team is expert Process Development, process optimization and scale-up to big quantity.
Business Type
Exporter, Importer, Manufacturer, Distributor, Supplier, Trading Company
Employee Count
26
Establishment
2013
Working Days
Sunday To Monday
GST NO
24AAKFN4352Q1ZR
Payment Mode
Cash Against Delivery (CAD), Cash on Delivery (COD), Cash Advance (CA), Cash in Advance (CID), Cheque, Days after Acceptance (DA), Delivery Point (DP), Letter of Credit (L/C), Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Paypal, Others
Certification
FIRM REGISTRATION
Related Products
Explore Related Categories
More Product From This seller
Seller Details

GST - 24AAKFN4352Q1ZR
Ankleshwar, Gujarat
Sales Department
Mr Sanjay Patel
Members since
12 Years
Address
Plot No. 4706/03, Gidc Estate, SF-12, Shrinathji Arcade, Near Meghmani Chowkadi, Ankleshwar, Gujarat, 393002, India
active pharmaceutical ingredients in Ankleshwar
Report incorrect details